Abstract
Purpose
The aim of this study was to investigate the safety and efficacy of transarterial chemoembolization and sorafenib (TACE-S) combined with microwave ablation (TACE-S-MWA) for the treatment of patients with advanced primary hepatocellular carcinoma (HCC).
Methods
Between January 2015 and December 2018, 152 consecutive advanced HCC patients, who underwent TACE-S-MWA (MWA group, n=77) or TACE-S (Non-MWA group, n=75), were investigated. Overall survival (OS), time to progression (TTP) and safety were compared between the two groups. Prognostic factors were analyzed using the Cox proportional hazard regression model.
Results
Baseline patient characteristics were balanced between the two groups. MWA group was associated with a higher OS (median, 19.0 vs 13.0 months; P<0.001) and a longer TTP (median, 6.0 vs 3.0 months; P<0.001) compared with non-MWA group. Multivariate analyses showed that portal vein tumor thrombosis (PVTT) (P=0.002), duration of sorafenib (P<0.001), and MWA treatment (P=0.011) were independently associated with OS. MWA treatment strategy (P<0.001) was a significant predictor of TTP. There were no treatment-related mortalities in either group. The rates of minor complications (42.9% vs 38.7%, P=0.599) and major complications (1.29% vs 1.33%, P=0.985) in the MWA group were similar to those in the non-MWA group.
Conclusion
TACE-S-MWA was safe and effective for advanced primary HCC. TACE-S-MWA resulted in better OS and TTP than did TACE-S for treatment of patients with advanced primary HCC.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, microwave ablation, sorafenib, survival
Introduction
Hepatocellular carcinoma (HCC) ranks the sixth most frequently diagnosed cancer and the third leading cause of cancer-related death in the world.1,2 HCC is associated with an overall poor prognosis despite the best diagnostic and therapeutic efforts. The Barcelona Clinic Liver Cancer (BCLC) classification has widely been used in the treatment of HCC. According to the BCLC guidelines, HCC patients with symptoms, vascular invasion, extrahepatic spread, or a combination are defined as advanced stage.3–5
Sorafenib is an oral multikinase inhibitor with antiproliferative and antiangiogenic effects by blocking the Ras/Raf/MEK/ERK signal transduction pathway. Furthermore, sorafenib can inhibit angiogenesis by targeting hepatocyte factor receptor (c-Kit), Fms-like tyrosine kinase (FLT-3), vascular endothelial growth factor (VEGF) receptor (VEGFR)-2, VEGFR-3, platelet-derived growth factor receptor (PDGFR-β) and other tyrosine kinases.6–8 The SHARP (Sorafenib HCC Assessment Randomized Protocol) trial showed that sorafenib was associated with a higher median overall survival (OS) (10.7 vs 7.9 months; P=0.0006) and a higher median time to progression (TTP) (5.5 vs 2.8 months; P=0.000007) compared with placebo group.9 Additionally, some other phase III randomized clinical trials reported that sorafenib was associated with significantly better clinical efficacy compared with other treatments for patients with advanced HCC.10–15
Sorafenib has long been recommended as a standard treatment for advanced HCC.3–5 Transarterial chemoembolization (TACE) has been validated as an effective treatment for unresectable HCC (BCLC stage B/C).3–5,16–18 However, the high recurrence rate after TACE treatment is a major limitation. Fortunately, some studies have shown that the combination of TACE and sorafenib (hereafter, TACE-S) can improve the OS of HCC patients with a better efficacy than either TACE or sorafenib monotherapy.19–23
Microwave ablation (MWA) is one of the most often used ablation treatments for liver tumor, lung cancer, renal cell carcinoma, or thyroid carcinoma.24–26 Compared with other ablation procedures, MWA was associated with more spherical and predictable ablation zones, less susceptible to the heat sink, and less dependent on its properties.27–30 As a palliative treatment, TACE was associated with a complete tumor necrosis rate of only 10–20%.31–34 Previous studies reported that TACE combined with MWA could improve tumor necrosis rate and enhance the median OS of patients with unresectable HCC.35–37 However, whether complementary MWA improves the outcomes of TACE-S in patients with advanced primary HCC remains unclear. In this study, we hypothesized that TACE-S combined with MWA (hereafter, TACE-S-MWA) could improve the clinical efficacy of TACE-S for advanced primary HCC. Therefore, the purpose of this study was to investigate the safety and efficacy of TACE-S-MWA in patients with advanced primary HCC.
Materials and Methods
Patients and Study Design
This retrospective study complied with the standards of the Declaration of Helsinki and obtained approval from the institutional review board of two medical centers (the Sun Yat-sen Memorial Hospital of Sun Yat-sen University and the Sun Yat-sen University Cancer Center, Guangdong China). Written informed consent was obtained prior to each treatment. All enrolled patients were diagnosed based on the criteria defined by the American Association for the Study of Liver Disease and the European Association for the Study of Liver. The advanced-stage HCC was defined according to the BCLC guidelines. Follow-up duration was terminated on June 30, 2019. From January 2015 to December 2018, we reviewed the medical data of 276 consecutive advanced HCC patients who underwent TACE-S or TACE-S-MWA. A total of 152 patients were ultimately enrolled, including 77 in the TACE-S-MWA group (MWA group) and 75 in the TACE-S group (Non-MWA group). All patients were informed of the advantages and disadvantages of those two treatment options, including treatment outcomes, treatment-related morbidities, and costs, and the patients chose the treatment on their own decision.
The inclusion criteria were listed as follows: (a) age of 18–75 years, (b) an Eastern Cooperative Oncology Group (ECOG) performance status score of no more than 2, (c) Child–Pugh class A or B liver disease, (d) BCLC stage C HCC, (e) fewer than five HCC lesions that were no greater than 10.0 cm in maximum diameter, (f) without a history of receiving liver transplantation or surgical resection, (g) without a history of receiving other interventional treatments (eg, 125I seed implantation, radiofrequency ablation, cryoablation or percutaneous ethanol injection), and (h) without serve coagulation dysfunction (eg, prothrombin activity < 40%, international normalized ratio > 1.26 and/or platelet count < 50×109/L).
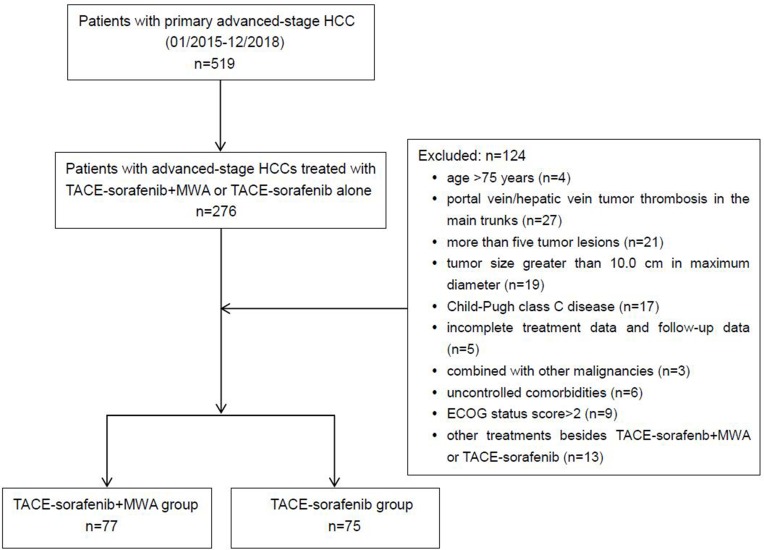
The exclusion criteria were listed as follows: (a) portal vein/hepatic vein tumor thrombosis in the main trunks, (b) history of encephalopathy or refractory ascites, (c) uncontrolled comorbidities (eg, general infection, serious dysfunction of heart or kidney, chronic obstructive pulmonary disease, or recent stroke), (d) other malignancies in addition to HCC, and (e) history of receiving systemic chemotherapy or immunotherapy (eg, programmed cell death-1/programmed cell death-ligand 1 antibody). The flowchart of the enrolled patients is demonstrated in Figure 1.
Figure 1.
Flowchart shows patient selection.
Abbreviations: HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; MWA, microwave ablation; ECOG, Eastern Cooperative Oncology Group.
Sorafenib Administration
All patients were given sorafenib since the first day of admission to the hospital. Initially, sorafenib treatment was suggested as a standard dose of 400 mg twice daily. Ideally, patients were treated with continuous sorafenib when TACE or MWA procedure was performed. In the case of the grade 3 or 4 sorafenib-related adverse events defined by the National Cancer Institute Common Terminology Criteria for Adverse Events occurred, the dose of sorafenib was reduced to 400 mg once daily. Patients were encouraged to insist on continuing the sorafenib treatment if the toxicity was manageable. Sorafenib was canceled if unmanageable treatment-related toxicity occurred or uncontrolled disease progression developed.
TACE Procedure
Digital subtraction angiography (DSA) (Allura Xper FD 20, Philips, Amsterdam, the Netherlands) was used for TACE procedure. Selective and superselective hepatic artery angiographies were performed with 5F catheter (RH or YASHIRO TPYE, Terumo, Tokyo, Japan) and 2.8F micro-catheter (Progreat, Terumo, Tokyo, Japan), respectively. After a general assessment of tumor factors, a lobaplatin solution (20–50 mg, 0.5 mg/mL) was injected via micro-catheter, followed by an emulsion of epirubicin (30–60 mg) mixed with Lipiodol (5–25 mL) (Lipiodol Ultrafluide, Guerbet, Aulnay-Sous-Bois, France). Finally, gelatin sponge particles (350–560 μm, Alicon, Hangzhou, China) mixed with contrast agent were administered into tumor-feeding arteries. The dosage of chemotherapeutics and embolization materials was based on the body weight and tumor status. The endpoint of TACE procedure was stasis of feeding arterial flow by post-embolization angiography.
MWA Procedure
MWA was performed percutaneously within 1–2 weeks after TACE. The timing of the followed MWA depended on the recovery of patients. After routine preparation, a plain computed tomography (CT) (SOMATOM 64 Sensation, Siemens, Muenchen, Germany) scan was first performed to confirm target tumor and puncture path. For small or medium HCC (maximum diameter < 5 cm), a single MWA electrode probe was inserted along the path to reach the opposite edge of tumor lesion through its center. For large HCC (5 ≤ maximum diameter ≤ 10 cm), multiple overlapping ablations were performed for accurate judgment of the required number of ablations, and the location of needle placement effectively reduced the tumor residual or recurrence. Local anesthesia and intravenous moderate sedation were performed during the procedure. The apparatus for MWA was ECO-100 water-cooled microwave apparatus (ECO Microwave Electronic Institute, Nanjing, China) and monopole microwave antenna (16–18G). The treatment parameters were set at 55–70 Watt and the procedure lasted for 5–15 mins. All MWA procedures were performed based on the manufacturer’s recommended protocol. After the MWA procedure, an immediate CT scan was performed to assess ablation zone and potential complications.
Assessment of Clinical Efficacy and Safety
OS was calculated from the diagnostic time of advanced HCC observed after initial treatment to the date of death or the last date of follow-up. Patients who remained alive at the date of the last follow-up were considered as “censored” in statistical analysis. TTP was defined as the interval between the diagnostic time of HCC observed after initial treatment and radiologic disease progression according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST). Time was censored at the date of death without progression and at the date of the last follow-up assessment in patients who were lost to follow-up. Complications or adverse events related to TACE, MWA or sorafenib were recorded during each treatment and follow-up period. Major complications were defined as events which caused substantial morbidity, or led to hospital admission, or prolonged the hospital stay. And the complications related to TACE or MWA procedure were evaluated according to the criteria defined by the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) classification system of complications.38
Follow-Up
Follow-up was conducted by clinical visits at monthly intervals. Physical examination, laboratory tests (eg, total bilirubin, serum albumin, prothrombin time, and serum tumor marker levels), and contrast-enhanced CT/magnetic resonance imaging (MRI) scan were performed. Tumor response was evaluated by contrast-enhanced CT/MRI scans every 4–6 weeks after each treatment. Local tumor status was assessed by our multidisciplinary team of radiologists and oncologists. If there was no tumor progression, the follow-up tests were prolonged to every 3 months. If residual tumor and/or tumor progression were observed, repeated MWA or TACE was performed based on a consensus decision made by our multidisciplinary team depending on the evaluation of tumor status by CT/MR imaging.
Statistical Analysis
All statistical analyses were performed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). The quantitative data was expressed as frequency, mean ± standard deviation (SD) or median with 95% confidence interval (CI). The Mann–Whitney U-test was applied to compare continuous variables, and either the Pearson’s χ2 test or the Fisher’s exact test was performed for comparing categorical data. Patients who remained alive at the date of the last follow-up were considered “censored”. Time was censored at the date of death without progression and at the date of the last follow-up assessment in patients who were lost to follow-up. Both cumulative OS and TTP were estimated by using the Kaplan–Meier method and were compared by using the Cox proportional hazards model. The statistically significant (P values less than 0.1) factors identified by the univariate analysis were entered into a Cox proportion hazards regression model to identify independent predictors of survival. For all tests, P-value of less than 0.05 was considered to be statistically significant.
Results
Patient Characteristics
Baseline patient characteristics were balanced between the two groups. Among all 152 patients, 136 (89.5%) were male, and the mean age was 54.2±12.21 years (range: 22–79 years) in the MWA group and 54.9±13.31 years (range: 27–78 years) in the non-MWA group. There were 46 patients in the MWA group and 49 patients in the non-MWA group with PVTT. The mean duration of sorafenib therapy was 14.2±9.11 months (range: 3–39 months) in the MWA group and 11.9±8.21 months (range: 3–28 months) in the non-MWA group. The mean tumor size was 5.9±2.05 cm (range: 3.7–10.0 cm) in the MWA group and 6.4±2.11 cm (range: 4.1–10.0 cm) in the non-MWA group. There were 56 (36.8%) and 96 (63.2%) patients with single and multiple tumor lesions, respectively. There were 101 (66.4%) patients with a hepatic function of Child–Pugh class A and 51 (33.6%) patients with a hepatic function of Child–Pugh class B. More details on the demographic characteristics of included patients are summarized in Table 1.
Table 1.
Baseline Patient Characteristics
| Characteristic | MWA Group | Non-MWA Group | P Value |
|---|---|---|---|
| (n=77) | (n=75) | ||
| Age (Year) | 0.704 | ||
| Mean±SD | 54.22±12.21 | 54.92±13.31 | |
| Range | 22–79 | 27–78 | |
| Gender | 0.386 | ||
| Male | 70(90.9) | 66(88.0) | |
| Female | 7(9.1) | 9(12.0) | |
| ECOG (Score) | 0.684 | ||
| 0 | 16(20.8) | 10(13.3) | |
| 1 | 39(50.6) | 34(45.3) | |
| 2 | 22(28.6) | 31(41.4) | |
| Etiology | 0.121 | ||
| HBV/HCV | 58(75.3) | 60(80.0) | |
| Other | 19(24.7) | 15(20.0) | |
| Tumor Size (cm) | |||
| Mean±SD | 5.95±2.05 | 6.36±2.11 | 0.234 |
| Range | 3.7–10.0 | 4.1–10.0 | |
| No. of Tumors | 0.498 | ||
| Single | 33(42.9) | 23(30.7) | |
| Multiple | 44(57.1) | 52(69.3) | |
| PVTT | 46(59.8) | 49(65.3) | 0.194 |
| Main portal vein | 0 | 0 | — |
| First-order PV branch | 21(27.3) | 27(36.0) | 0.247 |
| Second- or lower-order PV branches | 25(32.5) | 22(29.3) | 0.676 |
| AFP Level (ng/mL) | |||
| ≤ 400 | 38(49.4) | 43(57.3) | 0.163 |
| > 400 | 39(50.6) | 32(42.7) | |
| Child–Pugh Class | |||
| A | 53(68.8) | 48(64.0) | 0.832 |
| B | 24(31.2) | 27(36.0) | |
| BCLC Stage C | 77(100) | 75(100) | — |
| TACE Sessions | |||
| Mean±SD | 3.29±1.38 | 2.88±1.15 | 0.051 |
| Range | 1–8 | 1–5 | |
| Duration of Sorafenib (Month) | |||
| Mean±SD | 14.19±9.11 | 11.85±8.21 | 0.099 |
| Range | 3–39 | 3–28 |
Note: Unless otherwise indicated, data are the number of patients, with percentage in parentheses.
Abbreviations: SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; PV, portal vein; PVTT, portal vein tumor thrombosis; AFP, a-fetoprotein; HCV, hepatitis C virus; cm, centimeter; BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization; S, sorafenib; MWA, microwave ablation.
Safety
There were no treatment-related mortalities in either group. Post-embolization syndrome, abdominal pain and sorafenib-related toxic effects were the most common complications/adverse events. The most common minor complications were abdominal pain (16.4%), vomiting (11.8%), nausea (5.3%), new ascites (3.9%), and pleural effusion (3.3%). Two (1.3%) patients experienced major complications (liver abscess and hepatic artery hemorrhage), which resulted in prolonged hospital stay and additional post-procedure therapies. According to the CIRSE classification system of complications, the rates of grade 1, grade 2, and grade 3 complications, respectively, in the MWA group were similar to those, respectively, in the non-MWA group (grade 1: 24.7% vs 21.3%, P=0.669; grade 2: 18.2% vs 17.3%, P=0.891; grade 3: 1.29% vs 1.33%, P=0.985) (Table 2). There were no complications of grade 4, grade 5 or grade 6 in either group. Hand–foot skin reactions (38.8%), diarrhea (59.9%), fatigue (42.8%), hypertension (29.6%), alopecia (17.1%) and dysphonia (5.9%) were the most common sorafenib-related adverse events. There were no significant statistical differences between those two groups on complications/adverse events (Table 2).
Table 2.
Complications and Adverse Events Related to TACE, MWA, and Sorafenib
| Complications/AEs | MWA Group (n=77) | Non-MWA Group (n=75) | P Value |
|---|---|---|---|
| Minor Complications | 33(42.9) | 29(38.7) | 0.599 |
| Abdominal pain | 15(19.5) | 10(13.3) | — |
| Vomiting | 7(9.1) | 11(16.0) | — |
| Nausea | 5(6.5) | 3(4.0) | — |
| New ascites | 3(3.9) | 3(4.0) | — |
| Pleural effusion | 3(3.9) | 2(2.7) | — |
| Major Complications | 1(1.29) | 1(1.33) | 0.985 |
| Liver abscess | 0 | 1(1.33) | — |
| Hepatic artery hemorrhage | 1(1.29) | 0 | — |
| CIRSE Grade | |||
| 1 | 19(24.7) | 16(21.3) | 0.625 |
| 2 | 14(18.2) | 13(17.3) | 0.891 |
| 3 | 1(1.29) | 1(1.33) | 0.985 |
| 4–6 | — | — | — |
| Sorafenib Related AEs | |||
| Hypertension | 24(31.2) | 21(28.0) | 0.669 |
| Hand-foot skin reactions | 31(40.3) | 28(37.3) | 0.711 |
| Alopecia | 11(14.3) | 15(20.0) | 0.390 |
| Diarrhea | 43(55.8) | 48(64.0) | 0.305 |
| Gastrointestinal hemorrhage | — | — | — |
| Fatigue | 35(45.5) | 30(40.0) | 0.415 |
| Dysphonia | 5(6.5) | 4(5.3) | 0.762 |
Note: Unless otherwise indicated, data are the number of patients, with percentage in parentheses.
Abbreviations: TACE, transarterial chemoembolization; MWA, microwave ablation; AEs, adverse events; CIRSE, Cardiovascular and Interventional Radiological Society of Europe.
Clinical Efficacy
The clinical efficacy data are summarized in Table 3. In the MWA group, three patients experienced complete response (3.9%), 33 (42.8%) experienced partial response, 26 (33.8%) experienced stable disease and 15 (19.5%) experienced progressive disease. In the non-MWA group, zero patients experienced complete response (0%), 9 (12.0%) experienced partial response, 20 (26.7%) experienced stable disease and 46 (61.3%) experienced progressive disease. The disease control rate (complete response + partial response + stable disease) based on the mRECIST criteria was 80.5% in the MWA group and 38.7% in the non-MWA group (P<0.001).
Table 3.
Summary of Clinical Efficacy in MWA and Non-MWA Groups
| MWA Group | Non-MWA Group | P Value | |
|---|---|---|---|
| (n=77) | (n=75) | ||
| Tumor Response | |||
| CR | 3 (3.9) | 0 (0) | 0.245 |
| PR | 33 (42.8) | 9 (12.0) | <0.001 |
| SD | 26 (33.8) | 20 (26.7) | 0.341 |
| ORR (CR+PR) | 36 (46.7) | 9 (12.0) | <0.001 |
| DCR (CR+PR+SD) | 62 (80.5) | 29 (38.7) | <0.001 |
| TTP (Month)* | 6.0 (5.1–6.9) | 3.0 (2.7–3.3) | <0.001 |
| OS (Month)* | 19.0 (14.4–77.8) | 13.0 (11.6–14.4) | <0.001 |
Note: *Data are medians, with 95% confidence interval in parentheses.
Abbreviations: MWA, microwave ablation; CR, complete response; PR, partial response; SD, stable disease; ORR, overall response rate; DCR, disease control rate; TTP, time to progression; OS, overall survival.
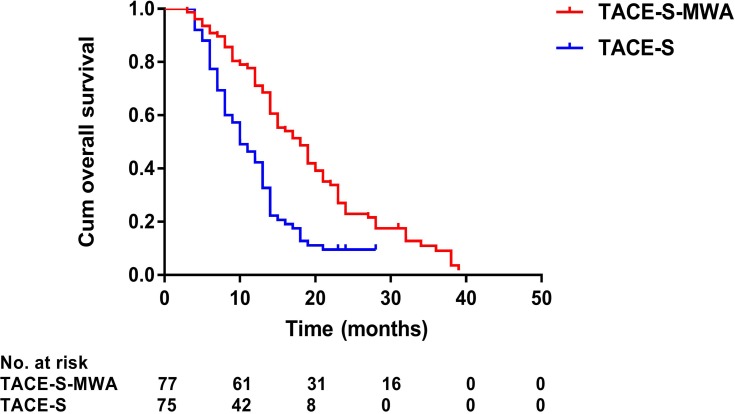
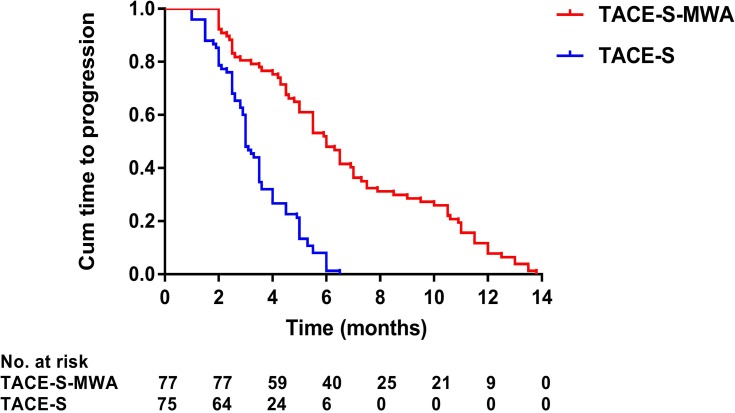
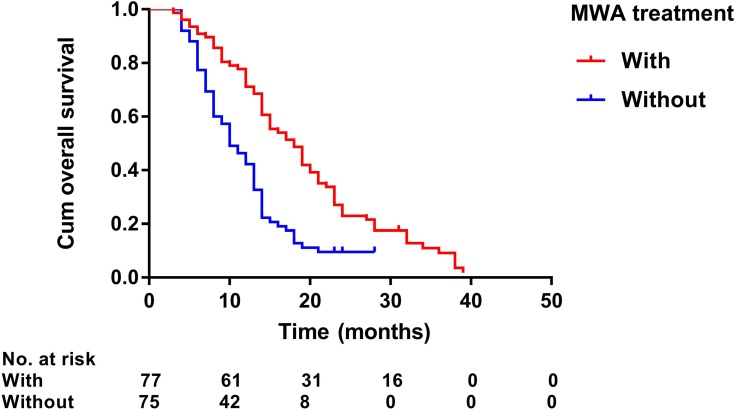
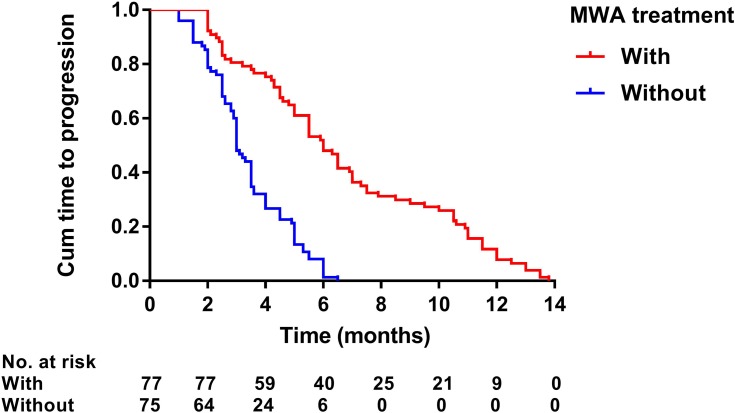
Median follow-up duration was 19.0 months (range: 3–39 months) in the MWA group and 13.0 months (range: 4–28 months) in the non-MWA group. After the follow-up duration, 136 patients had died, and 16 patients survived at their last visit. Median OS was 19.0 months (95% CI: 14.4–77.8 months) in the MWA group and 13.0 months (95% CI: 11.6–14.4 months) in the non-MWA group (P<0.001) (Figure 2). Median TTP was 6.0 months (95% CI: 5.1–6.9 months) in the MWA group and 3.0 months (95% CI: 2.7–3.3 months) in the non-MWA group (P<0.001) (Figure 3). The patients in the MWA group had a significantly higher OS (P<0.001) and longer TTP (P<0.001) than those patients in the non-MWA group.
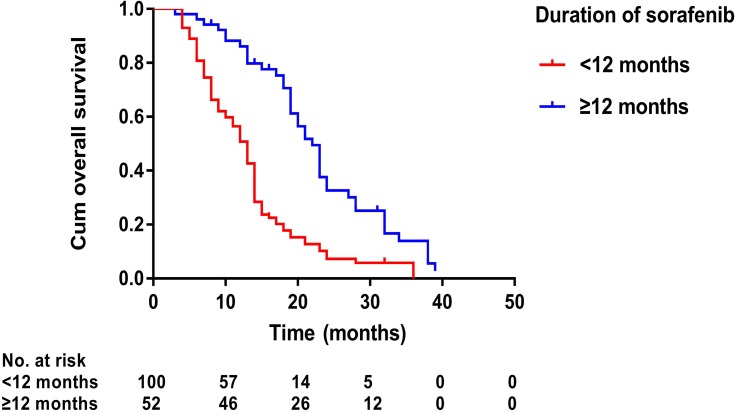
Figure 4.
Kaplan–Meier curves of overall survival in 152 patients with advanced primary hepatocellular carcinoma with sorafenib duration of no less than 12 months or less than 12 months.
Figure 2.
Kaplan–Meier curves of overall survival in 152 patients with advanced primary hepatocellular carcinoma treated with TACE-S-MWA or TACE-S.
Abbreviations: TACE, transarterial chemoembolization; MWA, microwave ablation; S, sorafenib.
Figure 3.
Kaplan–Meier curves of time to progression in 152 patients with advanced primary hepatocellular carcinoma treated with TACE-S-MWA or TACE-S.
Abbreviations: TACE, transarterial chemoembolization; MWA, microwave ablation; S, sorafenib.
Prognostic Factors Associated with OS and TTP
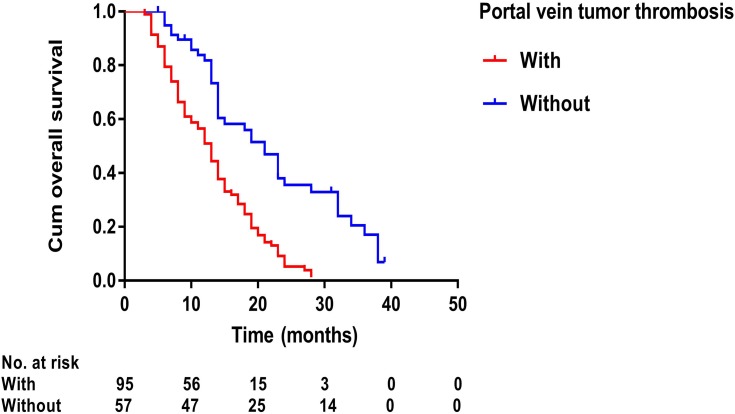
Prognostic factors of OS and TTP are shown in Table 4. Univariate analysis showed that ECOG performance status (P<0.001), number of tumors (P=0.002), presence of PVTT (P<0.001), Child–Pugh class (P=0.007), duration of sorafenib (P<0.001), TACE sessions (P=0.071), and MWA treatment (P<0.001) were associated with OS, whereas ECOG performance status (P=0.007), duration of sorafenib (P<0.001), TACE sessions (P=0.016), and MWA treatment (P<0.001) were associated with TTP. Multivariate analysis showed that the presence of PVTT (hazard ratio [HR] 2.08; 95% CI: 1.32, 3.28; P=0.002), duration of sorafenib (HR 2.31; 95% CI: 1.45, 3.67; P<0.001), and MWA treatment (HR 2.03; 95% CI: 1.18, 3.50; P=0.011) were independent predictors of OS (Figure 4–6) and that the MWA treatment (HR 3.64; 95% CI: 2.37, 5.60; P<0.001) was independently associated with TTP (Figure 7).
Table 4.
Prognostic Factors Associated with Overall Survival and Time to Progression
| Factor No. of Patients | Overall Survival | Time To Progression | ||||
|---|---|---|---|---|---|---|
| (n=152) | Univariate Analysis Multivariate Analysis Univariate Analysis Multivariate Analysis | |||||
| P Value | HR (95% CI) | P Value | P Value HR (95% CI) P Value | |||
| Age (Year) | 0.684 | 0.100 | ||||
| <65 | 99 | |||||
| ≥65 | 53 | |||||
| Gender | 0.103 | 0.900 | ||||
| Male | 136 | |||||
| Female | 16 | |||||
| ECOG (Score) | <0.001 | 0.71 (0.47–1.09) | 0.117 | 0.007 | 0.74 (0.52–1.07) 0.112 | |
| ≤1 | 99 | |||||
| 2 | 53 | |||||
| Etiology | 0.962 | 0.871 | ||||
| HBV/HCV | 118 | |||||
| None | 34 | |||||
| No. of Tumors | 0.002 | 0.76 (0.51–1.13) | 0.171 | 0.767 | ||
| Single | 56 | |||||
| Multiple | 96 | |||||
| Tumor Size | 0.278 | 0.165 | ||||
| ≤5 cm | 24 | |||||
| >5 cm | 128 | |||||
| PVTT | <0.001 | 2.08 (1.32–3.28) | 0.002 | 0.837 | ||
| Without | 57 | |||||
| With | 95 | |||||
| AFP Level | 0.235 | 0.856 | ||||
| ≤400 ng/mL | 81 | |||||
| >400 ng/mL | 71 | |||||
| Child–Pugh Class | 0.007 | 0.88 (0.57–1.37) | 0.582 | 0.689 | ||
| A | 101 | |||||
| B | 51 | |||||
| Duration of Sorafenib | <0.001 | 2.31 (1.45–3.67) | <0.001 | <0.001 | 1.14 (0.78–1.67) 0.488 | |
| <12 months | 100 | |||||
| ≥12 months | 52 | |||||
| TACE Sessions | 0.071 | 0.99 (0.68–1.46) | 0.979 | 0.016 | 1.08 (0.76–1.53) 0.687 | |
| ≤3 | 77 | |||||
| >3 | 75 | |||||
| MWA Treatment | <0.001 | 2.03 (1.18–3.50) | 0.011 | <0.001 | 3.64 (2.37–5.60) <0.001 | |
| Without | 75 | |||||
| With | 77 | |||||
Note: Data in parentheses are 95% confidence intervals.
Abbreviations: HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; cm, centimeter; PVTT, portal vein tumor thrombosis; AFP, a-fetoprotein; TACE, transarterial chemoembolization; MWA, microwave ablation.
Figure 5.
Kaplan–Meier curves of overall survival in 152 patients with advanced primary hepatocellular carcinoma with or without portal vein tumor thrombosis.
Figure 6.
Kaplan–Meier curves of overall survival in 152 patients with advanced primary hepatocellular carcinoma with or without microwave ablation treatment.
Figure 7.
Kaplan–Meier curves of time to progression in 152 patients with advanced primary hepatocellular carcinoma with or without microwave ablation treatment.
Discussion
Our data showed that TACE-S-MWA was safe and effective in the treatment of patients with advanced primary HCC. In this study, TACE-S-MWA resulted in a higher OS (median, 19.0 vs 13.0 months) and a longer TTP (median, 6.0 vs 3.0 months) compared with TACE-S. And the prognostic analyses showed that the presence of PVTT, sorafenib duration, and MWA treatments were independent predictors of OS. Furthermore, we found that TACE-S-MWA treatments were well tolerated in advanced HCC patients, and the rates of complications or adverse events in patients who underwent TACE-S-MWA were similar to those in patients who underwent TACE-S.
Few studies reported the clinical efficacy and safety of TACE-S-MWA in patients with advanced HCC. Zhu et al investigated the long-term outcomes of sorafenib combined with TACE and radiofrequency ablation (RFA) (S-TACE-RFA) in patients with medium or large (range: 3.1–7.0 cm in diameter) HCC.39 The researchers reported that the patients who underwent S-TACE-RFA had a longer RFS (median, 24.0 vs 10.0 months) and a higher OS (median, 63.0 vs 36.0 months) than those patients who underwent TACE-RFA.39 However, after reviewing the characteristics of their patients, we found that all of the patients were associated with BCLC stage A/B HCC. Therefore, it was inappropriate to compare the results of our study directly with those of their study due to the obvious differences in tumor status, performance status, or liver function. Peng et al reported the utilities of S-TACE-RFA in patients with advanced recurrent HCC.40 The study showed that OS (median, 14.0 vs 9.0 months) and TTP (median, 7.0 vs 4.0 months) were significantly longer in the S-TACE-RFA group than in the sorafenib monotherapy group.40 It indicated that our findings were similar to those in the previous studies.
The advantages of TACE-S-MWA for advanced HCC are due to the mutual benefits for the efficacy improvement of TACE, sorafenib and MWA treatments. TACE has long been considered as a standard treatment for unresectable HCC.3–5 The rationale of TACE was based on the fact that tumor growth mostly depended on the blood supply from the hepatic artery of HCC patients. However, the complete necrosis rate of target tumor after TACE was only 10–20%.31–34 The changes of tumor microenvironment after TACE played an important role in this phenomenon. Previous studies had shown that the overexpression of hypoxia-inducible factor-1 in hypoxic tumor microenvironment after TACE could obviously enhance the expression level of vascular endothelial growth factor (VEGF), and finally resulted in the proliferation of tumor cells.41,42 Hence, VEGF had appeared as a key role underlying the mechanism of hypoxia-induced neoangiogenesis and tumor progression. As a multikinase inhibitor, sorafenib could inhibit the synthesis of VEGF and the formation of new blood vessels. Therefore, sorafenib was believed to improve the clinical efficacy of TACE by decreasing the post-TACE angiogenesis and proliferation of hepatoma. In TACE-S treatment, sorafenib, as a complementary treatment acting on VEGF, could enhance the clinical efficacy by reducing the expression level of VEGF, when administrated sequentially after TACE. A previous phase II clinical trial showed that TACE combined with sorafenib was associated with a disease control rate that was up to 91.2%, and that the combination treatment considerably increased the survival time for intermediate- and advanced-stage HCC patients.43 Thus, TACE-S had been validated as an effective and safe treatment for patients with advanced HCC.
In the present study, our data showed that TACE-S-MWA resulted in reliable clinical outcomes for patients with advanced HCC. In comparison with TACE-S alone, TACE-S-MWA improved median OS and TTP from 13 to 19 months and 3 to 6 months, respectively. Previous studies reported that TACE combined with MWA (TACE-MWA) was associated with several advantages in patients with unresectable HCC.44–46 Firstly, liver dysfunction was a risk prognostic factor of long-term survival in HCC patients. It was previously shown that sustained TACE treatment was more likely to cause liver failure than the other local treatments or systemic treatments. Therefore, due to the negative effects of TACE treatment on liver function, the potentially required treatment had to be stopped in some patients, which might result in regrowth or metastases of target tumors. However, TACE-MWA could improve the long-term survival of these patients by reducing the repeated sessions of TACE procedure and protecting liver function indirectly. Secondly, because of the complicated tumor status and the palliative features of TACE procedure, it was hardly possible to achieve complete necrosis of target tumor during the treatment. However, the sequentially combined MWA after TACE could destroy the residual or recurrent tumor radically. Previous studies reported that TACE-MWA could enhance the local efficacy and prolong the long-term survival of patients. Thirdly, it had been confirmed that TACE could enlarge the ablation zone of MWA by reducing the “cooling effect” of intrahepatic blood flow and played an important role in inducing tumor destruction. Fourthly, hypo-vascular HCC and complicated feeding artery after TACE-S were the major limitations of TACE procedure. However, the sequentially followed MWA after TACE-S could destroy the target tumor directly. Additionally, molecular-targeted drugs such as sorafenib and apatinib were found to be effective for improving the clinical efficacy of local ablation in the treatment of HCC. Sun et al47 reported that the combination of radiofrequency ablation and sorafenib could prolong the median progression-free survival time further than RFA monotherapy for advanced-stage HCC (7.8 vs 4.6 months). Xie et al48 conducted an experimental study which showed that apatinib could inhibit the epithelial–mesenchymal transition of HCC cells and enhance the antitumor effect of RFA on HCC.
In terms of safety, both TACE-S and MWA are minimally invasive therapies. All interventional procedures were successfully performed. There were no treatment-related mortalities in this study. Because of the synergistic advantages of TACE-S and MWA, no patients suffered serious complications, such as serious thrombocytopenia, hyperbilirubinemia, hypoleukocytosis, or hepatic dysfunction. The rates of the CIRSE grade 1, grade 2, and grade 3 complications, respectively, of patients in the MWA group were similar to those, respectively, in the non-MWA group. There were no complications of the CIRSE grade 4, grade 5 or grade 6 in our study. Hand–foot skin reactions, diarrhea, fatigue, hypertension, alopecia and dysphonia were the most common sorafenib-related adverse events. There were no significant statistical differences between the two groups on complications or adverse events. It indicated that the rates of complications or adverse events in our study were similar to those in previous studies.39,40
To the best of our knowledge, this was the first study to provide the evidence of the comparison of TACE-S-MWA and TACE-S in patients with advanced HCC. Some potential limitations might exist in our study. First, this study was retrospective. However, the baseline demographics were matched well between the two groups, and we confirm that the data regarding survival and safety analyses were accurate and well recorded by our reviewers. Second, the included patients were associated with a tumor status of fewer than five HCC lesions that were no greater than 10.0 cm in maximum diameter. Patients with portal vein/hepatic vein tumor thrombosis in the main trunks were excluded. Further study is necessary to validate our results by a large, multi-center, and randomized controlled patient cohort. Third, the number of included patients was limited due to the novel treatment strategy of TACE-S-MWA in patients with advanced primary HCC.
In conclusion, TACE-S-MWA was safe and effective in patients with advanced primary HCC. TACE-S-MWA resulted in better clinical efficacy than did TACE-S in the patients. Further study is necessary to validate our results by a large, multi-center, and randomized controlled patient cohort.
Acknowledgments
This study was supported by the Scientific Research Foundation of Medical Science of Guangdong Province, China (grant number: A2018267), from Guangdong Science and Technology Department (2017B030314026), and was also supported by grant [2013]163 from Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology and grant KLB09001 from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes.
Funding Statement
This study was funded by the National Natural Science Foundation of China [grant number: 81901854] and the Scientific Research Foundation of Medical Science of Guangdong Province, China [grant number: A2018267].
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all individual participants in the study.
Disclosure
We declare that we have no conflicts of interest.
References
- 1.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041 [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Mokdad AA, Hester CA, Singal AG, et al. Management of hepatocellular in the United States. Chin Clin Oncol. 2017;6:21. doi: 10.21037/cco [DOI] [PubMed] [Google Scholar]
- 6.Roskoski R Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol Res. 2019;144:19–50. doi: 10.1016/j.phrs.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka S, Arii S. Molecular targeted therapies in hepatocellular carcinoma. J Semin Oncol. 2012;39:486–492. doi: 10.1053/j.seminoncol.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 8.Zhu YJ, Zheng B, Wang HY, et al. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38:614–622. doi: 10.1038/aps.2017.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 10.Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized Phase III trials. J Clin Oncol. 2017;35:622–628. doi: 10.1200/JCO.2016.69.5197 [DOI] [PubMed] [Google Scholar]
- 11.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 13.Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172–179. doi: 10.1200/JCO.2013.54.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372 [DOI] [PubMed] [Google Scholar]
- 15.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410 [DOI] [PubMed] [Google Scholar]
- 16.Pan T, Li XS, Xie QK, et al. Safety and efficacy of transarterial chemoembolization plus sorafenib for hepatocellular carcinoma with portal venous tumour thrombus. Clin Radiol. 2014;69:e553–e561. doi: 10.1016/j.crad.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 17.Liu PH, Lee YH, Hsia CY, et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol. 2014;21:1825–1833. doi: 10.1245/s10434-014-3510-3 [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Zhang C, Zhao Y, et al. Transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis: prognostic factors in a single-center study of 188 patients. Biomed Res Int. 2014;2014:194278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64:1090–1098. doi: 10.1016/j.jhep.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 20.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 21.Ohki T, Sato K, Yamagami M, et al. Efficacy of transcatheter arterial chemoembolization followed by sorafenib for intermediate/advanced hepatocellular carcinoma in patients in Japan: a retrospective analysis. Clin Drug Investig. 2015;35:751–759. doi: 10.1007/s40261-015-0333-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu H, Duan Z, Long X, et al. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. PLoS ONE. 2014;9:e96620. doi: 10.1371/journal.pone.0096620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Zhao W, Wang M, et al. Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18:138. doi: 10.1186/s12876-018-0849-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nour-Eldin NA, Exner S, Al-Subhi M, et al. Ablation therapy of non-colorectal cancer lung metastases: retrospective analysis of tumour response post-laser-induced interstitial thermotherapy (LITT), radiofrequency ablation (RFA) and microwave ablation (MWA). Int J Hyperthermia. 2017;33:820–829. doi: 10.1080/02656736.2017.1306656 [DOI] [PubMed] [Google Scholar]
- 25.Prins FM, Kerkmeijer LGW, Pronk AA, et al. Renal cell carcinoma: alternative nephron-sparing treatment options for small renal masses, a systematic review. J Endourol. 2017;31:963–975. doi: 10.1089/end.2017.0382 [DOI] [PubMed] [Google Scholar]
- 26.Korkusuz Y, Gröner D, Raczynski N, et al. Thermal ablation of thyroid nodules: are radiofrequency ablation, microwave ablation and high intensity focused ultrasound equally safe and effective methods? Eur Radiol. 2018;28:929–935. doi: 10.1007/s00330-017-5039-x [DOI] [PubMed] [Google Scholar]
- 27.Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37:2967–2973. doi: 10.1118/1.3432569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brace CL, Hinshaw JL, Laeseke PF, et al. Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology. 2009;251:705–711. doi: 10.1148/radiol.2513081564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillams AR, Lees WR. Radiofrequency ablation of lung metastases: factors influencing success. Eur Radiol. 2008;18:672–677. doi: 10.1007/s00330-007-0811-y [DOI] [PubMed] [Google Scholar]
- 30.Crocetti L, Bozzi E, Faviana P, et al. Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol. 2010;33:818–827. doi: 10.1007/s00270-010-9869-z [DOI] [PubMed] [Google Scholar]
- 31.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134 [DOI] [PubMed] [Google Scholar]
- 32.Wáng YX, De Baere T, Idée JM, et al. Transcatheter embolization therapy in liver cancer: an update of clinical evidences. Chin J Cancer Res. 2015;27:96–121. doi: 10.3978/j.issn.1000-9604.2015.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol. 2012;39:503–509. doi: 10.1053/j.seminoncol.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 34.Farinati F, Giacomin A, Vanin V, et al. TACE treatment in hepatocellular carcinoma: what should we do now? J Hepatol. 2012;57:221–222. doi: 10.1016/j.jhep.2011.12.022 [DOI] [PubMed] [Google Scholar]
- 35.Xu LF, Sun HL, Chen YT, et al. Large primary hepatocellular carcinoma: transarterialchemoembolization monotherapy versus combined transarterialchemoembolization-percutaneous microwave coagulationtherapy. J Gastroenterol Hepatol. 2013;28:456–463. doi: 10.1111/jgh.12088 [DOI] [PubMed] [Google Scholar]
- 36.Chen QF, Jia ZY, Yang ZQ, et al. Transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-microwave ablation therapy for hepatocellular carcinoma tumors ≤5 cm: a propensity analysis at a single center. Cardiovasc Intervent Radiol. 2017;40:1748–1755. doi: 10.1007/s00270-017-1736-8 [DOI] [PubMed] [Google Scholar]
- 37.Veltri A, Gazzera C, Calandri M, et al. Percutaneous treatment of Hepatocellular carcinoma exceeding 3 cm: combined therapy or microwave ablation? Preliminary results. Radiol Med. 2015;120:1177–1183. doi: 10.1007/s11547-015-0550-0 [DOI] [PubMed] [Google Scholar]
- 38.Filippiadis DK, Binkert C, Pellerin O, et al. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40:1141–1146. doi: 10.1007/s00270-017-1703-4 [DOI] [PubMed] [Google Scholar]
- 39.Zhu K, Huang J, Lai L, et al. Medium or large hepatocellular carcinoma: sorafenib combined with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;288:300–307. doi: 10.1148/radiol.2018172028 [DOI] [PubMed] [Google Scholar]
- 40.Peng Z, Chen S, Wei M, et al. Advanced recurrent hepatocellular carcinoma: treatment with sorafenib alone or in combination with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;287:705–714. doi: 10.1148/radiol.2018171541 [DOI] [PubMed] [Google Scholar]
- 41.Huang M, Wang L, Chen J, et al. Regulation of COX-2 expression and epithelial-to-mesenchymal transition by hypoxia-inducible factor-1α is associated with poor prognosis in hepatocellular carcinoma patients post TACE surgery. Int J Oncol. 2016;48:2144–2154. doi: 10.3892/ijo.2016.3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni JY, Xu LF, Wang WD, et al. Transarterial embolization combined with RNA interference targeting hypoxia-inducible factor-1α for hepatocellular carcinoma: a preliminary study of rat model. J Cancer Res Clin Oncol. 2017;143:199–207. doi: 10.1007/s00432-016-2237-x [DOI] [PubMed] [Google Scholar]
- 43.Chao Y, Chung YH, Han G, et al. Study in Asia of the combination of transarterial chemoembolization (TACE) with sorafenib in hepatocellular carcinoma trial (START): second interim safety and efficacy analysis. J Clin Oncol. 2010;28:4026. doi: 10.1200/jco.2010.28.15_suppl.4026 [DOI] [Google Scholar]
- 44.Hu H, Chen GF, Yuan W, et al. Microwave ablation with chemoembolization for large hepatocellular carcinoma in patients with cirrhosis. Int J Hyperthermia. 2018;34:1351–1358. doi: 10.1080/02656736.2018.1462536 [DOI] [PubMed] [Google Scholar]
- 45.Smolock AR, Cristescu MM, Hinshaw A, et al. Combination transarterial chemoembolization and microwave ablation improves local tumor control for 3- to 5-cm hepatocellular carcinoma when compared with transarterial chemoembolization alone. Abdom Radiol (NY). 2018;43:2497–2504. doi: 10.1007/s00261-018-1464-9 [DOI] [PubMed] [Google Scholar]
- 46.Ni JY, Liu SS, Xu LF, et al. Transarterial chemoembolization combined with percutaneous radiofrequency ablation versus TACE and PRFA monotherapy in the treatment for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:653–659. doi: 10.1007/s00432-012-1369-x [DOI] [PubMed] [Google Scholar]
- 47.Sun JJ, Zhao HJ, Li W. Clinical study of radiofrequency ablation therapy in combination with sorafenib for advanced hepatocellular carcinoma. J Clin Hepatol. 2011;27:1093–1098. [Google Scholar]
- 48.Xie H, Tian S, Yu H, et al. A new apatinib microcrystal formulation enhances the effect of radiofrequency ablation treatment on hepatocellular carcinoma. Onco Targets Ther. 2018;11:3257–3265. doi: 10.2147/OTT [DOI] [PMC free article] [PubMed] [Google Scholar]