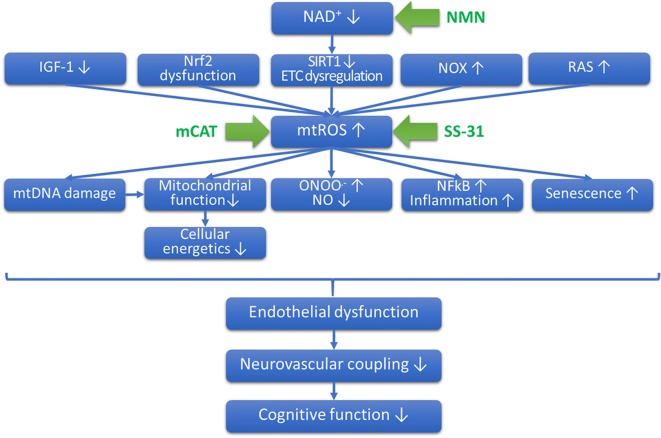
Fig. 2.
Proposed role for increased mitochondrial oxidative stress in cerebromicrovascular endothelial impairment and neurovascular dysfunction in aging. The scheme depicts that age-related decline in circulating IGF-1 (Csiszar et al. 2008), impairment of Nrf2-regulated antioxidant mechanisms (Tarantini et al. 2018a; Ungvari et al. 2018b; Dai et al. 2012; Bailey-Downs et al. 2012; Fulop et al. 2018; Ungvari et al. 2011a, b), decline in NAD+ levels and SIRT1 activity and consequential dysregulation of components of the electron transport chain (ETC) (Tarantini et al. 2019), upregulation of NADPH oxidases (NOX) (Park et al. 2007), and the local renin-angiotensin system (RAS) (Dai et al. 2012) act synergistically to promote increased mitochondrial production of reactive oxygen species (mtROS). Mitochondrial oxidative stress promotes mtDNA damage, exacerbating mitochondrial dysfunction, promotes peroxynitrite (ONOO.-) formation reducing bioavailability of NO, promotes NFkB activation (Ungvari et al. 2007), and induces endothelial senescence. All of these mechanisms contribute to endothelial dysfunction, which impair neurovascular coupling responses compromising cognitive function. The model predicts that diverse interventions that attenuate mitochondrial oxidative stress will confer endothelial protection, improving neurovascular coupling responses, thereby preserving cognitive function in old age. Accordingly, overexpression of catalase targeted to the mitochondria (mCAT), treatment with mitochondria-targeted antioxidants (SS-31), and sirtuin-activating treatments (e.g., the NAD+ booster nicotinamide mononucleotide [NMN]) attenuate mtROS production in endothelial cells, improving cerebromicrovascular endothelial function and rescuing neurovascular coupling responses