Figure 3. Rearrangements of trans-acting elements in the nucleotide-binding pocket.
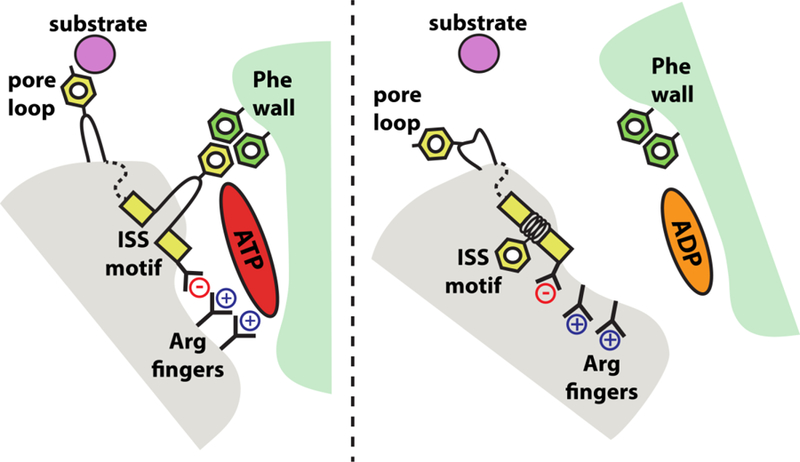
Schematic of the Yme1 nucleotide-binding pocket. When the cis subunit is bound to ATP, the gamma-phosphate is sensed by the trans arginine fingers, and the trans ISS motif extends across the pocket to form tight bridging interactions with cis phenylalanines. After hydrolysis to ADP, interactions between the arginine finger and the now absent gamma phosphate are lost, and the ISS motif winds back into the trans subunit, weakening the cis-trans subunit interface. These rearrangements are allosterically transmitted to the pore loops to break their interaction with the substrate polypeptide. Together, these motions allow the trans AAA+ domain to ‘step’ out from the spiral, unbound to substrate, and reset the ATPase ratchet.
