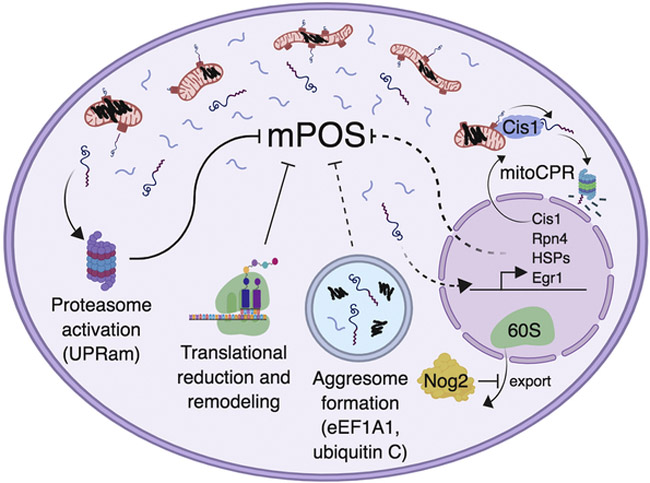
Figure 3. Cellular responses to mPOS.
IMM protein misfolding (black squiggles) causes mPOS (free floating blue lines surrounding mitochondria). mPOS activates proteasomal function (i.e. the unfolded protein response activated by mistargeting of proteins, UPRam), translational remodeling (e.g. reduced protein synthesis), and inhibition of nuclear export of the 60S ribosome by Nog2. When import is impaired, yeast increase transcription of Cis1, which then travels to the mitochondrial surface to facilitate the proteasomal degradation of unimported proteins, a process termed mitochondrial Compromised Protein import Response (mitoCPR). Rpn4 is similarly upregulated in yeast with deficient import, and then transcriptionally activates the proteasome to mitigate mPOS. We speculate (dotted lines) that the observed aggresome formation and increased transcription of Heat Shock Proteins (HSPs), Egr1 and its downstream targets play a role in alleviating mPOS in human cells. Current evidence suggests that eEF1A1 is involved in the formation of mitochondrial carrier-induced cytosolic aggresomes. (Created with BioRender.)