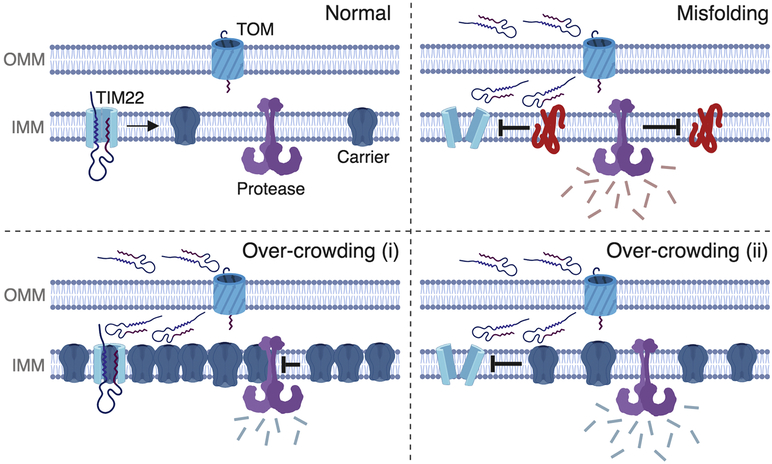
Figure 4. Potential mechanisms by which IMM carrier proteins may reduce protein import.
Four scenarios are depicted on the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM). Under normal circumstances (upper left), mitochondrial carrier proteins are imported by the TOM and the TIM22 complex, and then transferred laterally into the IMM where they co-exist with other proteins such as quality control proteases (depicted in purple). Upon IMM protein misfolding (upper right), the TTM22 translocase is destabilized and protein import is reduced to cause mPOS, as depicted by the unfolded proteins in the intermembrane space and cytosol. In the bottom two panels, we propose two possible mechanisms by which wild-type carrier protein over-crowding may cause reduce import: (i) it could cause a backup of the entire import process due to reduced ability to insert additional proteins into the protein-saturated membrane, and/or (ii) it could cause IMM proteostatic stress through ectopic protein-protein interactions and reduce import by destabilizing protein translocase complexes. (Created with BioRender.)