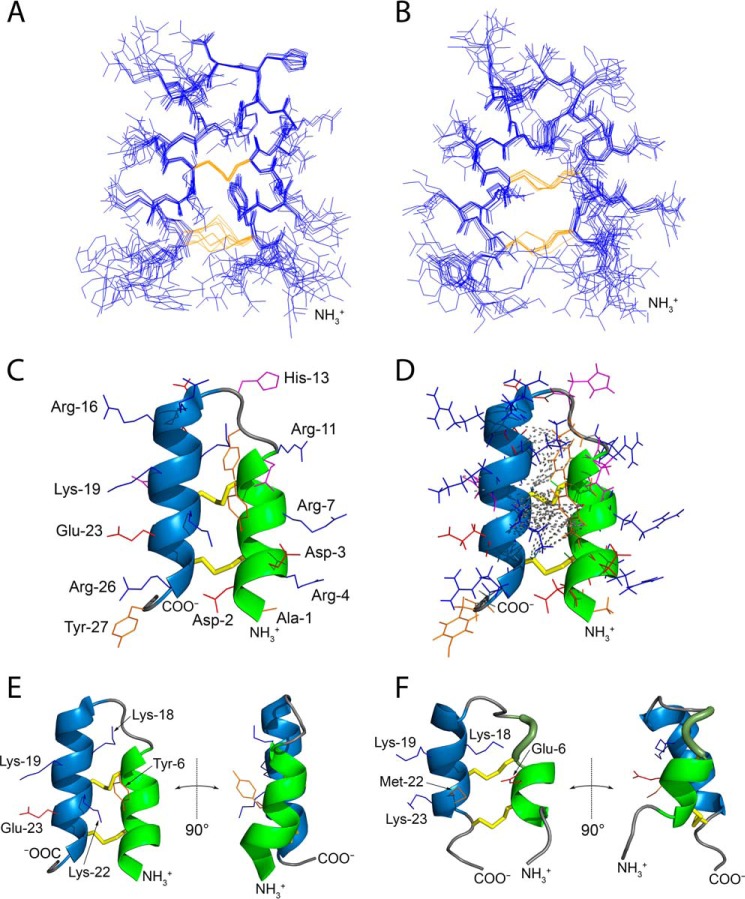
Figure 1.
Tk-hefu structure determined by NMR and its comparison with the parent peptide Tk-AMP-X2. A and B, ensembles of 10 independently derived NMR structures of Tk-hefu (A; PDB ID 5LM0) and Tk-AMP-X2 (B; PDB ID 2M6A) with the fewest restrain violations. N termini are labeled. Cystine side chains are in yellow. C, ribbon representation of Tk-hefu. The orientation of the molecule is as in A. The N-terminal α-helix is shown in green; C-terminal helix, sky blue; loops are presented as a gray string. S-S bonds are presented as yellow sticks, and other side chains are shown as lines; hydrophobic aliphatic and aromatic residues are orange; hydrophilic uncharged residues are magenta; positively charged residues, blue; and negatively charged, red. N and C termini and side chains are labeled. D, the same representation of Tk-hefu as in C but showing all atoms. Gray dashed lines denote the long-range NOESY connectivities between the protons of Tk-hefu. E and F, comparison of Tk-hefu (E) and Tk-AMP-X2 (F) structures. The color code is as in C, and the 310 helix in Tk-AMP-X2 is in smudge green. Side chains of residues discussed in the text are shown as lines and labeled. In C–F, the first structures from the set of 10 are presented (having the fewest restrain violations).