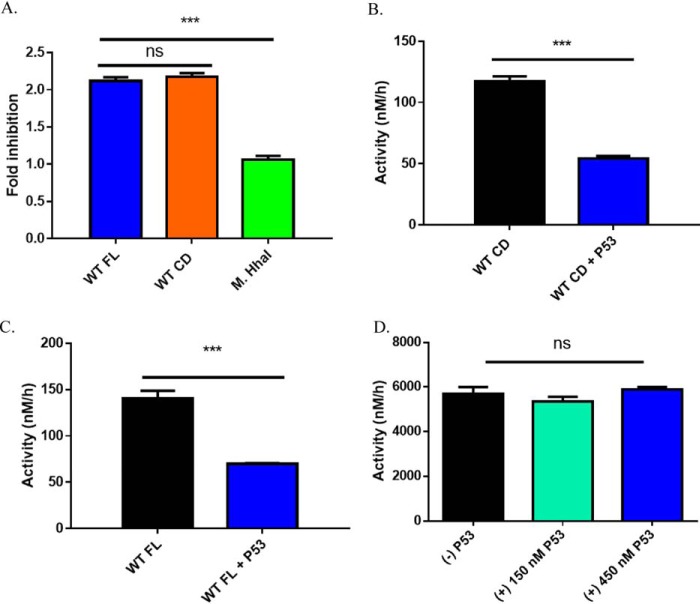
Figure 1.
p53WT-dependent inhibition of DNA methylation is specific to DNMT3A. A, fold-inhibition calculated by product formed by WT DNMT3A (full-length or catalytic domain enzymes) or M.HhaI divided by product formed by DNMT3A (full-length or catalytic domain enzymes) or M.HhaI without p53WT from reactions in B–D. Co-incubation of DNMT3A full-length (A, blue square calculated from B) and catalytic domain (A, orange square calculated from C) enzymes with p53WT (1:1 at 150 nm) leads to comparable levels of inhibition. Similar reactions involving the bacterial methyltransferase M.HhaI (A, green square calculated from D) with excess p53WT (1:3) failed to inhibit DNA methylation. In all co-incubations, proteins were held at 37 °C for 1 h prior to the start of the reaction by the addition of DNA (5 μm bp poly(dI-dC)). Data reflect the mean ± S.D. of 3 experiments; one-way analysis of variance was used to compare values of all three reactions; ***, p < 0.001; ns, p > 0.05.