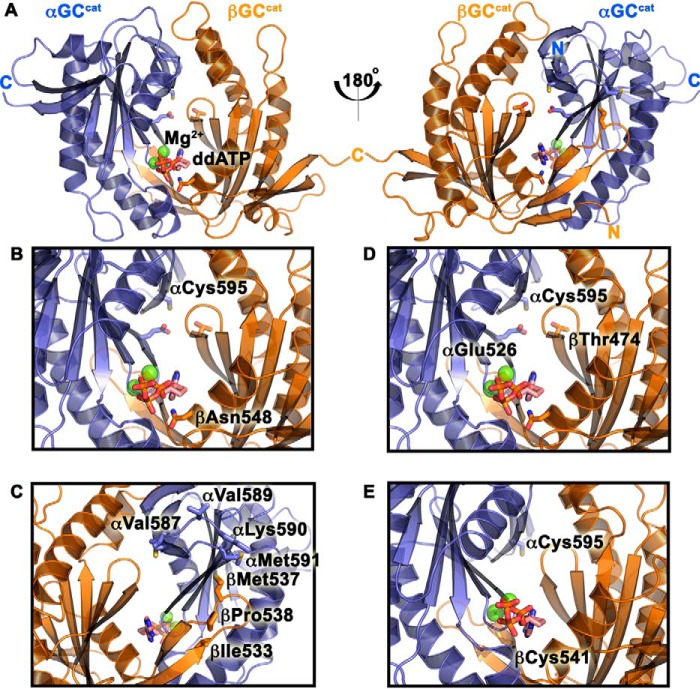
Figure 1.
Residues in the GC-1 catalytic domains targeted for mutagenesis. A, ventral (left) and dorsal (right) sides of the GC-1 catalytic domains (αβGCcat) in the modeled activated conformation with bound Mg2+ and dideoxy-ATP (ddATP) (13). B, residues αCys-595 and βAsn-548 were mutated to inactivate GC-1. C, dorsal flaps residues were mutated. D, residues αCys-595, αGlu-526, and βThr-474 were predicted to form a hydrogen-bond triad (13). E, substrate-binding residue βCys-541 was proposed to modulate substrate specificity (38).