Figure 5.
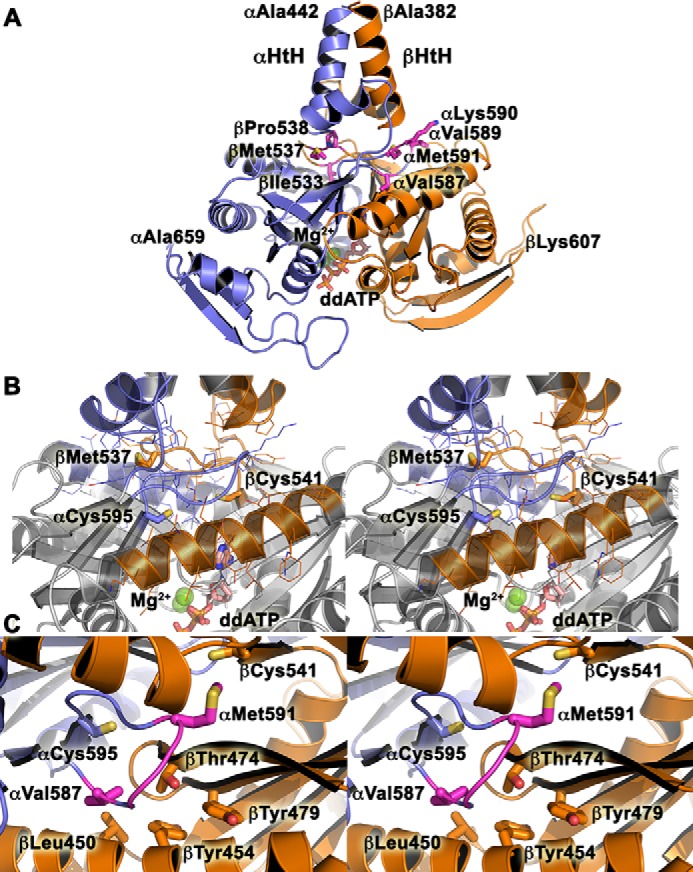
Model for WT CC-GCcat. A, the model for αGC-1 (442–659, blue) and βGC-1 (383–607, orange) was generated with SWISSMODEL (77, 78). Key residues from the dorsal flaps mutated in this study are shown as sticks and labeled. The helix-turn-helix (αHtH and βHtH) motifs of the penultimate coiled-coil domain are indicated. Magnesium ions (green balls) and dideoxy-ATP (sticks, ddATP) are included in the model. B, stereoview of the central positioning for the dorsal flaps, which are sandwiched between the helix-turn-helix motif and the active site of the catalytic domain. The view is the same as in A. Key residues are shown in sticks and colored in blue (αGC) and orange (βGC). C, stereoview of the hydrophobic pocket around residue βThr-474.
