Figure 6.
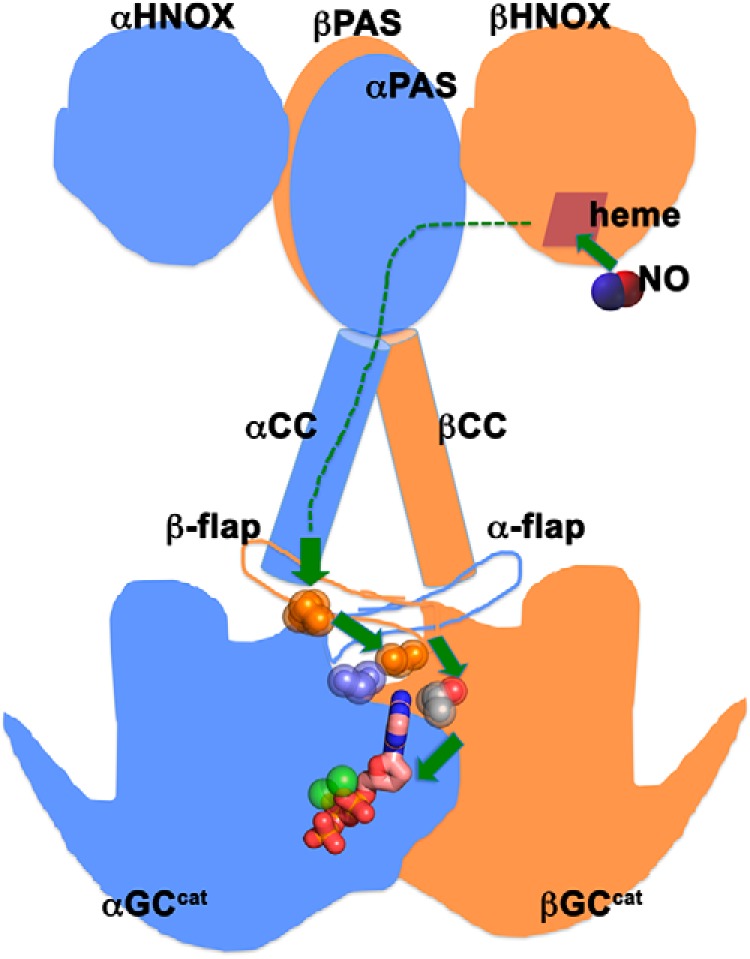
Proposed mechanism for sGC activation. Schematic representation of full-length sGC with the N-terminal HNOX domains, the dimerization domains (PAS), the coiled-coil domains (CC), and the C-terminal catalytic domains (GCcat). Upon NO binding to the HNOX heme, the activation signal gets transmitted through all the domains (dashed green lines). Hot spot residues (spheres) belonging to coupled networks in the dorsal flaps, the intersubunit interface, and the active site couple the preceding CC domains to the GCcat domains to promote activation.
