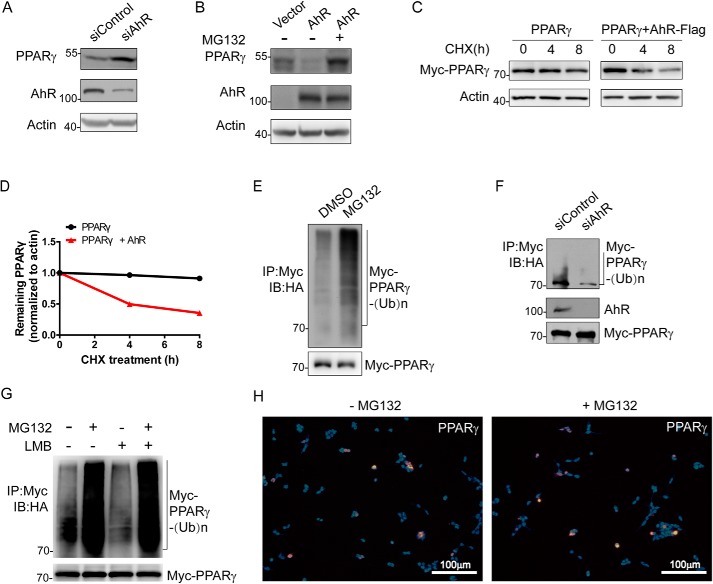
Figure 2.
AhR affects PPARγ protein level and stability. A, effect of AhR ablation on PPARγ protein level. 3T3-L1 cells were transfected with the indicated siRNAs (si) for 8 h. Cells were harvested, lysed, and immunoprecipitated with the indicated antibodies followed by Western blotting. B, effect of AhR overexpression on PPARγ protein level. 3T3-L1 cells were transfected with the indicated plasmids expressing AhR-Flag-His in the absence or presence of MG132. Cells were harvested, lysed, and immunoprecipitated with the indicated antibodies followed by Western blotting. C, effect of AhR on the protein stability of PPARγ. HEK293T cells were transfected with the indicated plasmids expressing Myc-PPARγ in the absence or presence of AhR-Flag-His for CHX-chase assay. Cells were harvested and lysed. The cell lysate was then detected with the indicated antibodies. D, turnover of PPARγ was determined by Western blotting. Signals from immunoblots were analyzed using Quantity One. PPARγ protein signals were normalized with the actin protein signals, and the percentage of PPARγ protein remaining was plotted against time. E, ubiquitination product mediated by CUL4B–AhR-associated complex was accumulated in the presence of proteasome inhibitor MG132. HEK293T cells were transfected with plasmids expressing Flag-Cul4B, Myc-PPARγ, AhR-Flag-His, and HA-Ub. Cells were then treated with DMSO or MG132 for 3 h. F, effect of CUL4B–AhR-associated complex on PPARγ ubiquitination. 3T3-L1 cells were transfected with the indicated siRNA for 5 h. The cells were then transfected with plasmids expressing Flag-Cul4B, Myc-PPARγ, and HA-Ub. After MG132 treatment for 4 h, cells were harvested, lysed, and immunoprecipitated with the indicated antibodies followed by Western blotting. G, HEK293T cells were transfected with plasmids expressing Flag-Cul4B, AhR-Flag-His, HA-Ub, and Myc-PPARγ. Cells were treated with MG132 for 3 h and LMB for 4 h followed by immunoprecipitation and Western blotting. Input (5%) was used for Western blotting. H, HEK293T cells were transfected with plasmids expressing Myc-PPARγ and AhR-Flag-His. Cells were treated with MG132 or DMSO for 6 h. Immunofluorescence against PPARγ was performed. Images were collected using a fluorescence microscope. IP, immunoprecipitation; IB, immunoblotting.