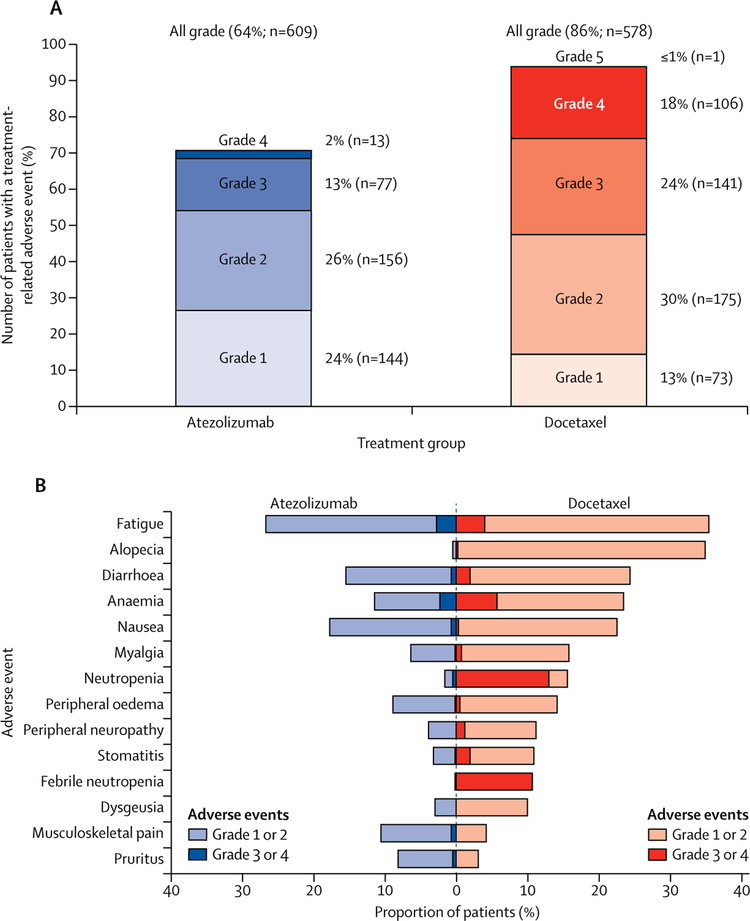
Figure 4: All-cause and treatment-related adverse events in the safety population.
(A) Adverse events that occurred within 30 days from the last study treatment were included in the analysis. Proportions of patients having treatment-related adverse events, by grade. (B) All-cause adverse events that differed by 5% or more between study groups. Additional adverse events with 5% or higher frequency in the docetaxel arm were not shown, and were neutrophil count decreased (10% vs <1% of patients in the docetaxel and atezolizumab arms, respectively), peripheral sensory neuropathy (7% vs 1%), mucosal inflammation (7% vs 1%), and nail disorder (5% vs 0%).