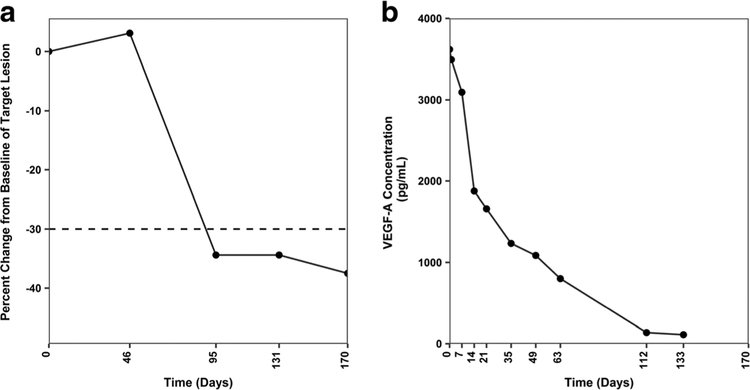
Fig. 3. Partial response to U3–1565 in a subject with metastatic colorectal cancer following treatment with U3–1565.
A 76 year old female with metastatic colorectal cancer treated with U3–1565 at 24 mg/kg/week (Part 1, Cohort 5) experienced a sustained PR. a Percent change from baseline as determined by the sum of longest diameters of the target lesion. The cutoff for PR (−30%) is shown by the dotted line. b This subject had a high baseline VEGF-A level (3619 pg/mL), which remarkably decreased over time upon U3–1565 treatment