Abstract
Introduction:
Alectinib demonstrated clinical efficacy and an acceptable safety profile in two phase II studies (NP28761 and NP28673). Here we report the pooled efficacy and safety data after 15 and 18 months more follow-up than in the respective primary analyses.
Methods:
Enrolled patients had ALK receptor tyrosine kinase gene (ALK)-positive NSCLC and had progressed while taking, or could not tolerate, crizotinib. Patients received oral alectinib, 600 mg twice daily. The primary end point in both studies was objective response rate assessed by an independent review committee (IRC) using the Response Evaluation Criteria in Solid Tumors, version 1.1. Secondary end points included disease control rate, duration of response, progression-free survival, overall survival, and safety.
Results:
The pooled data set included 225 patients (n = 138 in NP28673 and n = 87 in NP28761). The response-evaluable population included189 patients (84% [n = 122 in NP28673 and n = 67 in NP28761]). In the response-evaluable population, objective response rate as assessed by the IRC was 51.3% (95% confidence interval [CI]: 44.0–58.6 [all PRs]), the disease control rate was 78.8% (95% CI: 72.3–84.4), and the median duration of response was 14.9 months (95% CI: 11.1–20.4) after 58% of events. Median progression-free survival as assessed by the IRC was 8.3 months (95% CI: 7.0–11.3) and median overall survival was 26.0 months (95% CI: 21.4–not estimable). Grade 3 or higher adverse events (AEs) occurred in 40% of patients, 6% of patients had treatment withdrawn on account of AEs, and 33% had AEs leading to dose interruptions/modification.
Conclusions:
This pooled data analysis confirmed the robust systemic efficacy of alectinib in ALK-positive NSCLC with a durable response rate. Alectinib also had an acceptable safety profile with a longer duration of follow-up.
Keywords: Alectinib, Non–small cell lung cancer, NP28673, NP28761, Pooled analysis
Introduction
NSCLC harboring a chromosomal rearrangement of the anaplastic lymphoma kinase (ALK) gene (ALK-positive NSCLC), represents a distinct molecular subset of the disease, which affects approximately 5% of patients.1 Crizotinib is the current standard of care for ALK-positive NSCLC and has extended progression-free survival (PFS) compared with cytotoxic chemotherapy(10.9 months versus 7.7 months, respectively) in the first- and second-line treatment setting.2,3 Unfortunately, almost half of crizotinib-treated patients relapse within the first year. This is usually a result of poor control of disease within the central nervous system (CNS), which is the most common site of disease progression,4,5 or secondary ALK resistance mutations.6–8
Second-generation anaplastic lymphoma kinase (ALK) inhibitors have been developed with the aim of improving efficacy in patients with ALK-positive NSCLC, including those with CNS metastases. The ALK inhibitor ceritinib was granted accelerated approval by the U.S. Food and Drug Administration (FDA) in 2014 for use in patients with ALK-positive, metastatic NSCLC who had progressed while taking, or were intolerant of, crizotinib.9 The European Medicines Agency subsequently approved ceritinib in 2015 for use with the same indication.10 The approvals were based on a phase I and phase II study of ceritinib in patients with ALK-positive NSCLC, which demonstrated median PFS times of 5.7 to 6.9 months and objective response rates (ORRs) of 39% to 56%.11,12 Recently, the FDA approval was extended to treatment-naive patients with metastatic ALK-positive NSCLC.13 The extended approval was based on results from the ASCEND-4 trial, which demonstrated superior PFS with ceritinib versus with platinum-pemetrexed doublet chemotherapy in patients with treatment-naive, ALK-positive NSCLC (median PFS of 16.6 versus 8.1 months) (hazard ratio [HR] = 0.55, 95% confidence interval [CI]:0.42–0.73, p < 0.0001)14; a similar trend was observed in patients with CNS metastases at baseline, but it was not significant. ORRs were improved with ceritinib versus with chemotherapy in the overall study population (73% versus 27%) and in those with measurable CNS disease at baseline (46% versus 21%).14
Alectinib is a potent and highly selective ALK inhibitor that has demonstrated both systemic and CNS efficacy in ALK-positive NSCLC in a number of studies.15–18 Alectinib was approved in Japan in 2014 for the treatment of ALK inhibitor–naive patients with ALK-positive NSCLC after the results of a phase I/II study (AF001-JP). This study reported a high ORR of 93.5% (95% CI: 82–99); follow-up for this study is still ongoing, with a 3-year PFS rate of 62% (95% CI: 45–75).19 Similarly, significant clinical activity was reported with alectinib in two pivotal phase II studies, one global (NP28673 []) and one North American (NP28761 []), in patients with ALK-positive NSCLC who had previously received crizotinib. ORRs of 50.8% (95% CI: 41.6–60.0) and 52.2% (95% CI: 39.7–64.6) were observed in NP28673 and NP28761, respectively (data cutoff April 27, 2015), with a median duration of response (DOR) of 14.1 months (95% CI: 10.9–not estimable [NE] [44% of events] versus 13.5 months (95% CI: 6.7–NE [40% of events]), respectively. Alectinib was well tolerated in the global and North American studies, as reflected by the rates of dose interruptions (23% and 36%, respectively), dose reductions (10% and 16%, respectively), and withdrawals due to adverse events (AEs) (9% and 2%, respectively) reported (data cutoff date April 27, 2015).17,18 Data from these two phase II studies led to the accelerated approval of alectinib in 2015 by the FDA for treatment of patients with ALK-positive NSCLC who progressed while taking, or were intolerant of, crizotinib.20 Alectinib has also received conditional approval for the same patient population from the European Medicines Agency. Data from the first-line, phase III, global ALEX study demonstrated that patients treated with alectinib had a longer PFS than patients treated with crizotinib.21
Here, we present pooled efficacy and safety analyses from these phase II studies with 15 and 18 months more follow-up than in the respective primary analyses for NP28761 (data cutoff of January 22, 2016 versus October 24, 2014) and NP28673 (data cutoff of February 1, 2016 versus August 18, 2014).
Methods
Study Design
NP28673 and NP28761 were phase II, single-arm, open-label, multicenter studies. NP28673 was conducted across 16 countries at 56 sites and patients were enrolled between June 20, 2013, and April 23, 2014. NP28761 was undertaken in 27 centers across the United States and Canada, with patients enrolled between May 3, 2012, and August 4, 2014. This time frame also included a phase I dose-finding step; hence, the phase II portion of the study commenced on September 4, 2013. Both studies were undertaken in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines, and written informed consent was obtained from all patients. The full methodology for each study has been published previously.17,18
Eligibility Criteria
Both studies enrolled patients who were 18 years or older with locally advanced or metastatic ALK-positive NSCLC, as assessed by an FDA-approved fluorescence in situ hybridization test. Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2 or lower and had progressed while taking crizotinib. Patients with asymptomatic baseline CNS metastases (treated or untreated with radiation) and those who had received prior chemotherapy were permitted to enroll in both studies. Patients were excluded if they had received prior ALK inhibitor treatment other than crizotinib.
Study Treatment
All patients received 600 mg of alectinib orally twice daily with a meal, until disease progression, unacceptable toxicity, withdrawal, or death. In both studies there was a minimum washout period of 7 days between the last dose of crizotinib and the first dose of alectinib.
Study End Points
The primary end point of the pooled analysis was ORR assessed by an independent review committee (IRC) using the Response Evaluation Criteria in Solid Tumors, version 1.1. The secondary end points for both studies included disease control rate (DCR), DOR, PFS, overall survival (OS), and safety. CNS secondary end points were also evaluated, including CNS ORR and CNS DOR, and will be reported in a separate analysis.
Statistical Analysis
Response end points were assessed in the response-evaluable (RE) population, which comprised patients with measurable disease at baseline who received at least one dose of alectinib. The safety population comprised all patients who received at least one dose of alectinib. ORR was defined as the proportion of patients in the RE population who achieved a best overall response of confirmed complete response (CR) or partial response (PR). PFS and OS were assessed in the safety population. PFS was calculated from the date of first dose of alectinib until disease progression or death. OS was calculated from the date of first dose of alectinib until death. Time-to-event data (PFS, OS, and DOR) were estimated by Kaplan-Meier analyses.
Results
Patients
The pooled data set comprised 225 patients (138 patients from the study NP28673 and 87 patients from the study NP28761) (Supplementary Fig. 1). The RE population according to the IRC included 189 patients (84%), comprising 122 patients from NP28673 and 67 patients from NP28761. Baseline characteristics were similar across both studies (Table 1). Briefly, the median patient age was 53 years (range 22–79), 67% of patients had an ECOG PS of 1 or 2, and most patients (74%) were white. Overall, 136 patients (60%) had baseline CNS metastases and 174 (77%) had received prior chemotherapy (Table 1).
Table 1.
Demographic and Baseline Characteristics of the Pooled Population (ITT Population)
| Characteristic | NP28761 (n = 87) | NP28673 (n = 138) | Difference between Cohorts, % | Pooled Population (N = 225) |
|---|---|---|---|---|
| Median age (range), y | 54 (29–79) | 52 (22–79) | 2 y | 53 (22–79) |
| Sex, n (%) | ||||
| Male | 39 (45) | 61 (44) | 1 | 100 (44) |
| Female | 48 (55) | 77 (56) | 1 | 125 (56) |
| ECOG PS, n (%) | ||||
| 0 | 30 (34) | 44 (32) | 2 | 74 (33) |
| 1 | 48 (55) | 81 (59) | 4 | 129 (57) |
| 2 | 9 (10) | 13 (9) | 1 | 22 (10) |
| Race, n (%) | ||||
| White | 73 (84) | 93 (67) | 17 | 166 (74) |
| Asian | 7 (8) | 36 (26) | 18 | 43 (19) |
| Other | 3 (3) | 4 (3) | 0 | 7 (3) |
| Black/African American | 3 (3) | 1 (0.7) | 2.3 | 4 (2) |
| Multiple | 1 (1) | 0 (0) | 1 | 7 (3) |
| Unknown | 0 | 3 (2) | 2 | 1 (0.4) |
| American Indian/Alaska native | 0 | 1 (0.7) | 0.7 | 1 (0.4) |
| CNS metastases, n (%) | ||||
| Yes | 52 (60) | 84 (61) | 1 | 136 (60) |
| No | 35 (40) | 54 (39) | 1 | 89 (40) |
| Histologic subtype, n (%) | ||||
| Adenocarcinoma | 82 (94) | 133 (96) | 2 | 215 (96) |
| Other | 5 (6) | 5 (4) | 2 | 10 (4) |
| Prior chemotherapy, n (%) | ||||
| Yes | 64 (74) | 110 (80) | 6 | 174 (77) |
| No | 23 (26) | 28 (20) | 6 | 51 (23) |
| Crizotinib + prior therapies | ||||
| Crizotinib only | 23 (26) | 28 (20) | 6 | 51 (23) |
| +1 therapy | 0 | 52 (38) | 38 | 52 (23) |
| +2 therapies | 19 (22) | 16 (12) | 10 | 35 (16) |
| +3 therapies | 18 (21) | 17 (12) | 9 | 35 (16) |
| +4 therapies | 14 (16) | 16 (12) | 4 | 30 (13) |
| +5 therapies | 8 (9) | 4 (3) | 6 | 12 (5) |
| ≥6 therapies | 5 (6) | 5 (4) | 2 | 10 (4) |
| Smoking status | ||||
| Active smoker | 0 | 3 (2) | 2 | 3 (1) |
| Past smoker | 33 (38) | 39 (28) | 10 | 72 (32) |
| Never-smoker | 54 (62) | 96 (70) | 8 | 150 (67) |
ITT, intent-to-treat; ECOG, Eastern Cooperative Oncology Group; PS, performance status; CNS, central nervous system.
Efficacy
At the data cutoff (February 1, 2016, for NP28673 and January 22, 2016, for NP28761), the median follow-up for the pooled data set was 18.8 months (range 0.6–29.7). In the RE population, the ORR assessed by the IRC was 51.3% (95% CI: 44.0–58.6), with 97 of 189 patients achieving a PR; there were no CRs. Stable disease was reported in 52 of 189 patients (28%), giving a DCR of78.8% (95% CI: 72.3–84.4). The median DOR was 14.9 months (95% CI: 11.1–20.4) after 58% of events.
Of the patients who had received prior chemotherapy in the RE population (n ¼ 148), 73 (49%) achieved a PR; there were no CRs, giving an IRC-assessed ORR of 49.3% (95% CI: 41.0–57.7). In total, 44 of 148 patients (30%) had stable disease, resulting in a DCR of 79.1% (95% CI:71.6–85.3). The median DOR (based on 59% of events) in this subgroup was also 14.9 months (95% CI: 11.0–21.9).
Overall, 24 of 41 chemotherapy-naive patients in the RE population (59%) achieved a PR; there were no CRs, giving an IRC-assessed ORR of 58.5% (95% CI:42.1–73.7). Stable disease was reported in eight of 41 patients (20%) giving a DCR in this population of 78.0% (95% CI: 62.4–89.4). The median DOR was 11.2 months (95% CI: 8.0–NE) after 54% of events.
A subgroup analysis of IRC-assessed ORR was performed to evaluate different prognostic factors, including sex, race, ECOG PS, CNS metastases at baseline, smoking status, and prior chemotherapy. ORR rates were generally consistent across most subgroups. Patients with an ECOG PS of 0 had a numerically higher response rate compared with patients with an ECOG PS of 1 or 2(65.6% [95% CI: 52.3–77.3] versus 45.0% [95% CI: 35.6–54.8] or 41.2% [95% CI: 18.4–67.1], respectively). The analysis also showed a higher response rate in patients who were never-smokers at baseline than in those who were past smokers (55.9% [95% CI: 46.8–64.7] versus 39.0% [95% CI: 26.5–52.6], respectively) (Table 2). However, it should be noted that the subgroups were relatively small and confidence intervals were overlapping.
Table 2.
Subgroup Analyses of IRC Objective Response Rate in the Pooled Population (IRC RE Population)
| Responders per Subgroup | |||
|---|---|---|---|
| Characteristic | Patients per Subgroup (n = 189) | n (%) | 95% CI |
| Sex | |||
| Male | 88 | 46 (52.3) | 41.4–63.0 |
| Female | 101 | 51 (50.5) | 40.4–60.6 |
| Race | |||
| White | 137 | 70 (51.1) | 42.4–59.7 |
| Asian | 38 | 23 (60.5) | 43.4–76.0 |
| Other | 14 | 4 (28.6) | 8.4–58.1 |
| ECOG PS at baseline | |||
| 0 | 61 | 40 (65.6) | 52.3–77.3 |
| 1 | 111 | 50 (45.0) | 35.6–54.8 |
| 2 | 17 | 7 (41.2) | 18.4–67.1 |
| CNS metastases at baseline | |||
| Yes | 113 | 55 (48.7) | 39.2–58.3 |
| No | 76 | 42 (55.3) | 43.4–66.7 |
| Prior chemotherapy | |||
| Yes | 148 | 73 (49.3) | 41.0–57.7 |
| No | 41 | 24 (58.5) | 42.1–73.7 |
| No. of prior regimens | |||
| 1 or 2 | 89 | 48 (53.9) | 43.0–64.6 |
| 3–9 | 100 | 49 (49.0) | 38.9–59.2 |
| Smoking status at screening | |||
| Active smoker | 3 | 3 (100.0) | 29.2–100.0 |
| Past smoker | 59 | 23 (39.0) | 26.5–52.6 |
| Never-smoker | 127 | 71 (55.9) | 46.8–64.7 |
| Time receiving prior crizotinib | |||
| ≤Median | 105 | 48 (45.7) | 36.0–55.7 |
| ≥Median | 84 | 49 (58.3) | 47.1–69.0 |
| Best response to crizotinib | |||
| Complete response | 1 | 1 (100) | 2.5–100.0 |
| Partial response | 84 | 50 (59.5) | 48.3–70.1 |
| Stable disease | 43 | 19 (44.2) | 29.1–60.1 |
| Progressive disease | 47 | 21 (44.7) | 30.2–59.9 |
| Unknown, N/A, or NE | 14 | 6 (42.9) | 17.7–71.1 |
IRE, independent review committee; RE, response evaluable; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status; CNS, central nervous system; N/A, not applicable; NE, not evaluable.
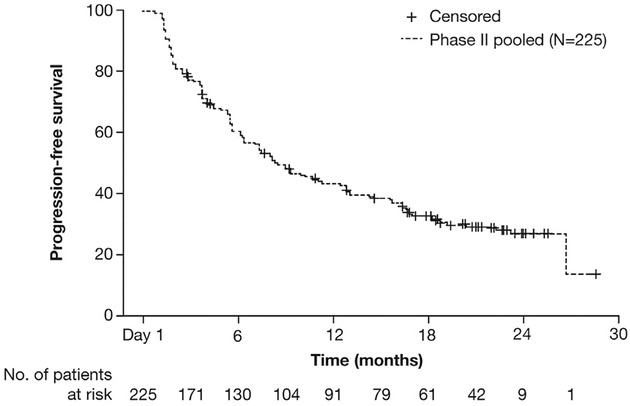
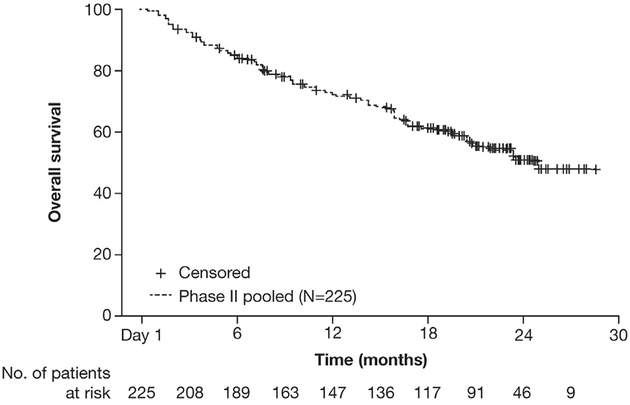
In the pooled population, 156 of 225 patients (69%) had a PFS event according to the IRC at the data cutoff. The median PFS was 8.3 months (95% CI: 7.0–11.3) (Fig. 1) and the 6-month event-free rate was 59.9% (95% CI: 53.5–66.4). For patients who had received only crizotinib treatment before receiving alectinib (51 of 225 [23%]), the median PFS was 8.4 months (95% CI: 5.6–16.6). With regard to OS, 96 of 225 patients (43%) had an OS event at the data cutoff. The median OS was 26.0 months (95% CI: 21.4–NE) and the 6-month event-free rate was 85.3% (95% CI: 80.6–89.9) (Fig. 2).
Figure 1.
Independent review committee–assessed progression-free survival of the pooled population (intent-to-treat population [N = 225]).
Figure 2.
Overall survival of the pooled population (intent-to-treat population [N = 225]).
Safety
Safety was evaluated in the pooled safety population of 225 patients (138 patients from the study NP28673 and 87 patients from the study NP28761). The mean dose intensity of alectinib was 94.1%.
AEs occurring at a frequency of more than 20% (any grade) were constipation (38%), fatigue (34%), peripheral edema (28%), myalgia (25%), nausea (23%), cough (21%), and headache (21%). A summary of AEs occurring at a frequency of more than 10% are shown in Table 3. Grade 3 to 5 AEs occurred in 40% of patients; the most common were dyspnea (4%), elevated levels of blood creatine phosphokinase (4%), alanine transaminase (3%), and aspartate transaminase (3%). Seven patients (3%) died during the study, including two of hemorrhage and one each of dyspnea, endocarditis, intestinal perforation, pulmonary embolism, and an unspecified cause. Only two deaths (1%) were considered by the investigator to be treatment related (hemorrhage and intestinal perforation).
Table 3.
AEs with an Incidence Rate Higher Than 10% in the Pooled Studies (ITT Population)
| AE, n (%) | NP28761 (n = 87) | NP28673 (n = 138) | Difference between Cohorts, % | Pooled Population (N = 225) |
|---|---|---|---|---|
| Patients with ≥1 AE | 84 (97) | 135 (98) | 1 | 219 (97) |
| Constipation | 32 (37) | 53 (38) | 1 | 85 (38) |
| Fatigue | 33 (38) | 43 (31) | 7 | 76 (34) |
| Peripheral edema | 22 (25) | 41 (30) | 5 | 63 (28) |
| Myalgia | 22 (25) | 35 (25) | 0 | 57 (25) |
| Nausea | 21 (24) | 30 (22) | 2 | 51 (23) |
| Cough | 18 (21) | 30 (22) | 1 | 48 (21) |
| Headache | 21 (24) | 26 (19) | 5 | 47 (21) |
| Diarrhea | 20 (23) | 22 (16) | 7 | 42 (19) |
| Dyspnea | 17 (20) | 23 (17) | 3 | 40 (18) |
| Increased aspartate transaminase level | 18 (21) | 18 (13) | 8 | 36 (16) |
| Anemia | 17 (20) | 16 (12) | 8 | 33 (15) |
| Weight increased | 16 (18) | 17 (12) | 6 | 33 (15) |
| Asthenia | 2 (2) | 30 (22) | 20 | 32 (14) |
| Upper respiratory tract infection | 13 (15) | 19 (14) | 1 | 32 (14) |
| Vomiting | 11 (13) | 21 (15) | 2 | 32 (14) |
| Increased alanine transaminase level | 16 (18) | 15 (11) | 7 | 31 (14) |
| Rash | 8 (9) | 22 (16) | 7 | 30 (13) |
| Back pain | 10 (11) | 18 (13) | 2 | 28 (12) |
| Increased blood bilirubin level | 9 (10) | 18 (13) | 3 | 27 (12) |
| Increased blood creatine phosphokinase level | 20 (23) | 6 (4) | 19 | 26 (12) |
| Dizziness | 11 (13) | 15 (11) | 2 | 26 (12) |
| Photosensitivity reaction | 10 (11) | 16 (12) | 1 | 26 (12) |
| Arthralgia | 10 (11) | 15 (11) | 0 | 25 (11) |
| Insomnia | 11 (13) | 12 (9) | 4 | 23 (10) |
| Decreased appetite | 5 (6) | 17 (12) | 6 | 22 (10) |
| Upper abdominal pain | 4 (5) | 17 (12) | 7 | 21 (9) |
| Nasopharyngitis | 3 (3) | 16 (12) | 9 | 19 (8) |
| Increased blood alkaline phosphatase level | 12 (14) | 5 (4) | 10 | 17 (8) |
| Hypokalemia | 9 (10) | 7 (5) | 5 | 16 (7) |
| Oropharyngeal pain | 2 (2) | 14 (10) | 8 | 16 (7) |
| Hypertriglyceridemia | 11 (13) | 0 | 13 | 11 (5) |
AE, adverse event; ITT, intent-to-treat.
AEs leading to dose modification or interruptions occurred in 33% of patients (n = 75), whereas AEs leading to treatment withdrawal were reported in 6% of patients (n = 14) (Table 4).
Table 4.
AEs Leading to Dose Modification, Interruption, or Withdrawal in the Pooled Studies (ITT Population)
| Outcome, n (%) | NP28761 (n = 87) | NP28673 (n = 138) | Pooled Population (N = 225) |
|---|---|---|---|
| AE leading to withdrawal from study | 2 (2) | 12 (9) | 14 (6) |
| AE leading to withdrawal from treatment | 2 (2) | 12 (9) | 14 (6) |
| AE leading to dose modification or interruption | 37 (43) | 38 (28) | 75 (33) |
| Serious AE leading to withdrawal from treatment | 1 (1) | 8 (6) | 9 (4) |
| Serious AE leading to dose modification or interruption | 9 (10) | 13 (9) | 22 (10) |
| Related AE leading to withdrawal from treatment | 2 (2) | 8 (6) | 10 (4) |
| Related AE leading to dose modification or interruption | 24 (28) | 23 (17) | 47 (21) |
AE, adverse event; ITT, intent-to-treat.
Discussion
Alectinib has demonstrated clinical systemic and CNS efficacy in two pivotal phase II trials, achieving high response rates and durable responses.17,18 In the present analysis, efficacy and safety data were pooled from these phase II trials, with 15 and 18 months more follow-up for NP28761 and NP28673, respectively. These data confirmed the clinical activity and acceptable safety profile of alectinib in patients with ALK-positive NSCLC after treatment with crizotinib.
Despite the differences in standard of care for ALK-positive NSCLC between the United States and the rest of the world, the patient populations in NP28761 and NP28673 were very similar, with 80% and 74% of patients progressing during prior chemotherapy and crizotinib therapy, respectively. Other baseline characteristics were also very similar across the two studies, including patient age (median 54 versus 52 years), proportion of male patients (45% versus 44%), patients with an ECOG PS of 0 or 1 (90% versus 91%), and patients with baseline CNS disease (60% versus 61%) in the North American and global studies, respectively, supporting the rationale for combining these data sets.
The ORR of 51.3% that we observed in the present analysis is consistent with the ORRs reported in the individual primary and updated analyses of NP28673 (49.2% and 50.8%, respectively) and NP28761 (47.8% and 52.2%, respectively).17,18 In this pooled analysis, alectinib demonstrated efficacy regardless of prior treatment with chemotherapy, with an ORR of 49.3% for patients who received prior chemotherapy compared with 58.5% for patients who were chemotherapy-naive.
Overall, the safety profile of alectinib in this pooled analysis was consistent with the data reported in the primary publications.17,18 Alectinib was well tolerated and most of the AEs were grade 1/2 in severity, with only 1% of deaths reported as being treatment related. During the pooling of these study data, exposure-response analysis was also performed. Multivariate logistic regression and Cox proportional hazards analyses of the efficacy data demonstrated no statistically significant relationship between alectinib exposure and best overall response or PFS across the two studies, and logistic regression analysis demonstrated no statistically significant relationship between alectinib exposure and safety end points.22 These exploratory analyses confirm that the alectinib dosing regimen of 600 mg twice daily provides exposures within the expected plateau range of response, supporting its selection as the global dosing regimen.
Crizotinib was the first ALK inhibitor to be approved for the treatment of ALK-positive NSCLC and it is the current standard of care. Crizotinib prolongs PFS, increases ORR, and provides a greater improvement in global quality of life compared with chemotherapy in both previously treated and treatment-naive, ALK-positive NSCLC.2,3 Ceritinib was also approved for the treatment of crizotinib-pretreated patients with ALK-positive NSCLC after achieving ORR rates of 39% to 56% and a median PFS of 5.7 to 6.9 months in phase I and II studies.11,12 Recently, ceritinib was also approved in the first-line setting for patients with ALK-positive NSCLC on the basis of PFS and ORRs superior to those with chemotherapy reported in the ASCEND-4 trial.14 TheORR and PFS for ceritinib are comparable with those of alectinib in this pooled analysis, but in the ASCEND-2 trial,12 ceritinib was associated with high rates of dose interruptions (76%) and modifications or discontinuations (54%). In contrast, alectinib demonstrated an acceptable safety profile and good tolerability in this pooled analysis, as reflected by the rate of dose interruptions and modifications (33%) and the low withdrawal rate (6%). A recent study of the ALK inhibitor brigatinib in the same setting as the two alectinib studies presented here showed an ORR of 45% to 54% and median PFS of 9.2 to 12.9 months with doses of 90 mg once daily or 90 mg once daily for 7 days followed by 180 mg once daily, respectively. Compared with alectinib, brigatinib showed comparable rates of dose reductions (7%) and dose interruptions (18%) due to AEs at the lower dose; however, at the higher dose brigatinib showed greater rates of dose reductions (20%), dose interruptions (36%), and discontinuations (8%).23
Here we have reported the systemic efficacy and safety of the pooled population; in addition, an analysis of the activity of alectinib on CNS metastases in this pooled data set has recently been published.24 Alectinib achieved a CNS ORR of 64.0% (95% CI: 49.2–77.1) with a CNS DCR of 90.0% (95% CI: 78.2–96.7) and CNS DOR of 10.8 months (95% CI: 78.2–90.8), showing good CNS efficacy.
Two ongoing phase III studies are directly comparing the efficacy of alectinib with crizotinib in patients with ALK inhibitor–naive ALK-positive NSCLC (ALEX [] and J-ALEX [JapicCTI-132316]). Following an interim analysis, results from the J-ALEX study were released early, as the primary end point of PFS demonstrated superiority compared with the PFS with crizotinib treatment (HR = 0.34 [99.6826% CI:0.17–0.70, stratified log-rank p < 0.0001]; median PFS not reached [95% CI: 20.3–NE] versus 10.2 months [95% CI: 8.2–12.0] for alectinib versus crizotinib).24,25 Grade 3 or 4 AEs were observed at a greater frequency in the crizotinib arm (52%) than in the alectinib arm (27%), and rates of drug interruptions were lower with alectinib than with crizotinib (29% versus 74%, respectively). Primary data from the global ALEX study also showed that alectinib had a PFS superior to that with crizotinib (12-month event-free survival rate, 68.4% [95% CI: 61.0–75.9] with alectinib versus 48.7% [95% CI: 40.4–56.9] with crizotinib).21
In conclusion, the results from this pooled analysis showed that alectinib, 600 mg twice daily, demonstrated clinical activity and was well tolerated in patients with ALK-positive NSCLC who had progressed while taking crizotinib. Efficacy was shown in patients who had received prior chemotherapy, as well as in those who were chemotherapy naive.
Supplementary Material
Acknowledgments
The authors would like to thank the patients, their families, and the participating study centers. Dr. Popat acknowledges National Health Service funding to the National Institute for Health Research Biomedical Research Centre at The Royal Marsden and the Institute of Cancer Research. Third-party medical writing assistance, under the direction of the authors, was provided by Louise Clarke of Gardiner-Caldwell Communications and was funded by F. Hoffmann-La Roche, Ltd.
Footnotes
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at http://dx.doi.org/10.1016/j.jtho.2017.06.070.
Disclosure: Dr. Yang has received advisory board fees from Boehringer Ingelheim, Bayer, AstraZeneca, Roche/Genentech, Chugai, Clovis Oncology, Eli Lilly, Merck Sharp and Dohme, Merck Serono, Pfizer, Novartis, Celgene, Merrimack, Yuhan Pharmaceuticals, and Daiichi Sankyo. Dr. Ou has received personal fees for Pfizer, AstraZeneca, ARIAD, and Roche outside the submitted work. Dr. De Petris has received personal fees from Roche, AstraZeneca, and Bristol-Myers Squibb. Dr. Gadgeel has received consultancy fees from Boehringer Ingelheim, ARIAD, Novartis, and Genentech. Dr. Gandhi has received consultancy fees from Genentech/Roche, Pfizer, Merck, Abbvie, and AstraZeneca and personal fees from Merck and Bristol-Myers Squibb IION Foundation. Dr. Kim has received personal fees from Roche. Dr. Barlesi has received consulting fees from Roche. Dr. Govindan has received travel accommodation fees and consulting fees from Merck, Boehringer Ingelheim, Celgene, Roche, Stemcentrix, and Abbe Vie and consultancy fees from GlaxoSmithKline, Clovis, and Helsinn Healthcare. Dr. Dingemans has received consultancy fees from Eli Lilly, AstraZeneca, Clovis, Boehringer Ingelheim, and Merck Sharp and Dohme. Dr. Lena reports advisory board membership for Roche, Merck Sharp and Dohme, Bristol-Myers Squibb, Novartis, Pfizer, and AstraZeneca and has been reimbursed for meeting expenses from Roche, Merck Sharp and Dohme, Bristol-Myers Squibb, Lilly, and Amgen. Dr. Popat has received personal fees from Roche, Pfizer, and Novartis outside the submitted work. Dr. Dansin has received personal fees from Bristol-Myers Squibb, AstraZeneca, and Roche. Ms. Golding, Dr. Bordogna, Dr. Balas, Mr. Morcos, and Dr. Zeaiter are employees of and have stock ownership in Roche. Dr. Shaw has received consulting fees from Ignyta and Taiho and advisory board fees from Pfizer, Novartis, Genentech/Roche, Ariad, Daiichi-Sankyo, Blueprint Medicines, Loxo, EMD Serono, and Foundation Medicine. The remaining authors declare no conflict of interest.
Previously published in abstract form as Yang et al. Pooled efficacy and safety data from two phase II studies (NP28673 and NP28761) of alectinib in ALK-positive non-small-cell lung cancer (NSCLC) [abstract]. J Thorac Oncol. 2017;12:S1170–S1171.
References
- 1.Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24:2371–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. [DOI] [PubMed] [Google Scholar]
- 3.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. [DOI] [PubMed] [Google Scholar]
- 4.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4:120ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–1739. [DOI] [PubMed] [Google Scholar]
- 9.Khozin S, Blumenthal GM, Zhang L, et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015;21:2436–2439. [DOI] [PubMed] [Google Scholar]
- 10.Novartis. Novartis lung cancer drug Zykadia gains EU approval, providing new therapy for certain patients with ALK + NSCLC. https://www.novartis.com/news/media-releases/novartis-lung-cancer-drug-zykadia%C2%AE-gains-eu-approval-providing-new-therapy. Accessed June 1, 2017.
- 11.Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mok T, Spigel D, Felip E, et al. ASCEND-2: a single-arm, open-label, multicenter phase II study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with chemotherapy and crizotinib (CRZ) [abstract]. J Clin Oncol. 2015;33(suppl): 8059. [Google Scholar]
- 13.U.S. Food and Drug Administration. FDA broadens ceritinib indication to previously untreated ALK-positive metastatic NSCLC. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560873.htm. Accessed June 1, 2017.
- 14.Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. [DOI] [PubMed] [Google Scholar]
- 15.Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14:590–598. [DOI] [PubMed] [Google Scholar]
- 16.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–1128. [DOI] [PubMed] [Google Scholar]
- 17.Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661–668. [DOI] [PubMed] [Google Scholar]
- 18.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura T, Kiura K, Seto T, et al. Three-year follow-up of an alectinib phase I/II study in ALK-positive non-small-cell lung cancer: AF-001JP. J Clin Oncol. 2017;35:1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. FDA approves new oral therapy to treat ALK-positive lung cancer. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm476926.htm. Accessed March 9, 2017.
- 21.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer [e-pub ahead of print]. N Engl J Med. 10.1056/NEJMoa1704795, accessed July 19, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Hsu JC, Carnac R, Henschel V, et al. Population pharmacokinetics (popPK) and exposure-response (ER) analyses to confirm alectinib 600 mg BID dose selection in a crizotinib-progressed or intolerant population [abstract]. J Clin Oncol. 2016;34(suppl):e20598. [Google Scholar]
- 23.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib (BRG) in patients (pts) with crizotinib (CRZ)-refractory ALK non-small cell lung cancer (NSCLC): first report of effiþcacy and safety from a pivotal randomized phase (ph) 2 trial (ALTA) [abstract]. J Clin Oncol. 2016b;34(suppl): 9007. [Google Scholar]
- 24.Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34:4079–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.