Figure 3.
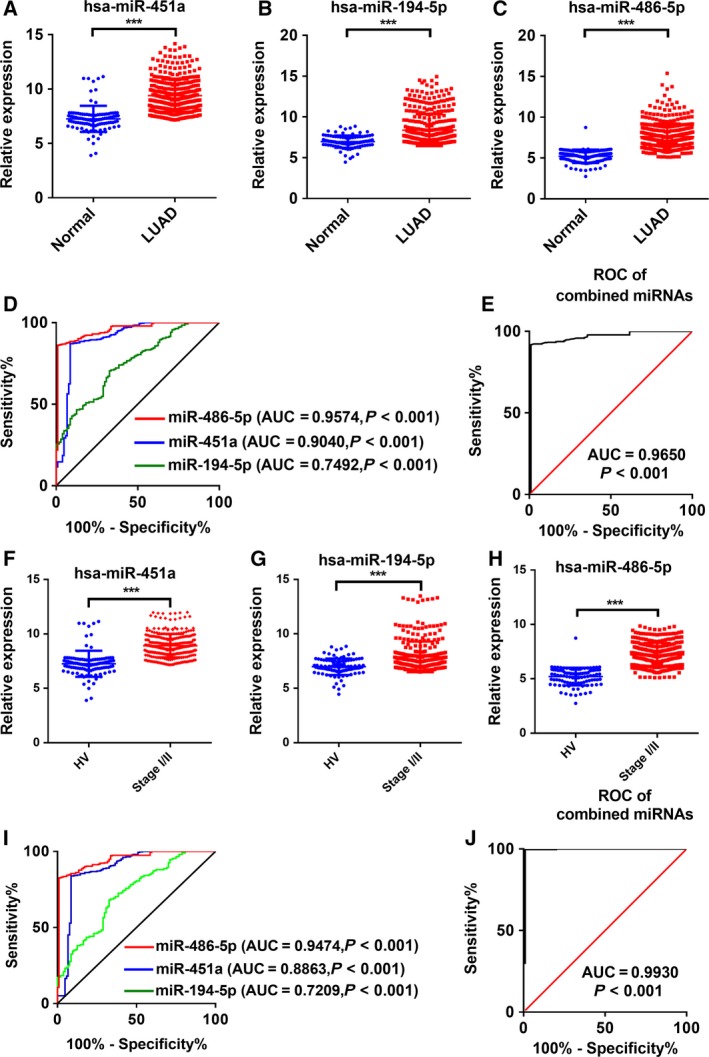
Plasma EV miRNA expression signature for LUAD diagnosis in the validation phase. (A–C) The expression of miR‐451a, miR‐194‐5p, and miR‐486‐5p in the plasma EVs from patients with LUAD (N = 389) and normal samples from healthy volunteers (N = 104) by qRT‐PCR. (D) ROC curve analysis for miR‐451a, miR‐194‐5p, and miR‐486‐5p in plasma EVs from patients with LUAD (N = 389) and normal samples from healthy volunteers (N = 104). (E) ROC curve analysis for the combined miRNA panel in plasma EVs from patients with LUAD (N = 389) and normal samples from healthy volunteers (N = 104). (F–H) The expression of miR‐451a, miR‐194‐5p, and miR‐486‐5p in plasma EVs from patients with stage I/II LUAD (N = 310) (stage I/II) and normal samples from healthy volunteers (N = 104) (HV) by qRT‐PCR. (I) ROC curve analysis for miR‐451a, miR‐194‐5p, and miR‐486‐5p in plasma EVs from patients with stage I/II LUAD (N = 310) and normal samples from healthy volunteers (N = 104). (J) ROC curve analysis for the combined miRNA panel in plasma EVs from patients with stage I/II LUAD (N = 310) and normal samples from healthy volunteers (N = 104). Each value is the mean ± SD; ***P < 0.001.
