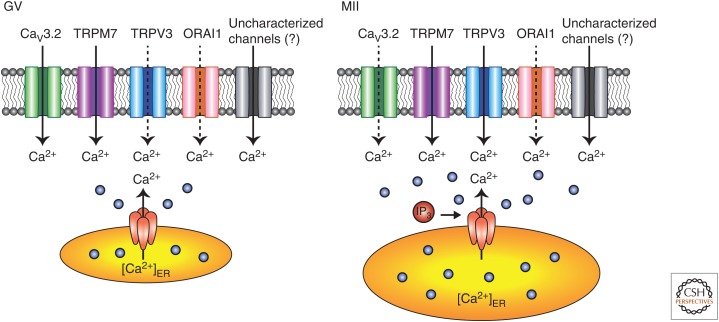
Figure 2.
Differential expression of plasma membrane (PM) Ca2+ channels in mammalian oocytes/eggs. Ca2+ influx plays a pivotal role in both spontaneous Ca2+ oscillations in germinal vesicle (GV) oocytes (left) and fertilization-induced oscillations in MII eggs (right). During oocyte maturation, the putative active channel(s) are progressively inactivated, which causes down-regulation of Ca2+ influx and termination of spontaneous Ca2+ oscillations by the GV breakdown stage, thereby avoiding parthenogenesis at the MII stage. A high [Ca2+]ER in MII eggs is important for robust IP3-induced Ca2+ release at the time of fertilization. Note that a variety of Ca2+ channels have been functionally detected in the PM of mouse oocytes, including the low-voltage-activated (LVA) T-type calcium channel 3.2 (Cav3.2 T-type), transient receptor potential (TRP) family members vanilloid member 3 (TRPV3), and TRP melastatin 7 (TRPM7) as well as ORAI1 channels. These channels are proposed to mediate the majority of the influx, although the PM channels that mediate Ca2+ influx during oocyte maturation and fertilization remain to be fully identified, and may be different between these two stages. In mouse GV oocytes, the persistently low [Ca2+]ER levels serve as the natural trigger for Ca2+ influx, which appears mostly operated through Cav3.2 channels. All the other channels are likely also to mediate additional influx, although of lesser magnitude. The only channel whose functional expression clearly increases during maturation in mouse oocytes is TRPV3, as it is nearly absent at the GV stage with its maximal expression being at the MII stage. TRPM7 also seems to change during maturation, as there is higher current density in GV oocytes than in MII eggs. However, fertilization reactivates influx, although how this is accomplished is presently unknown. Thick complete arrows, active influx; broken arrows, reduced expression and/or function.