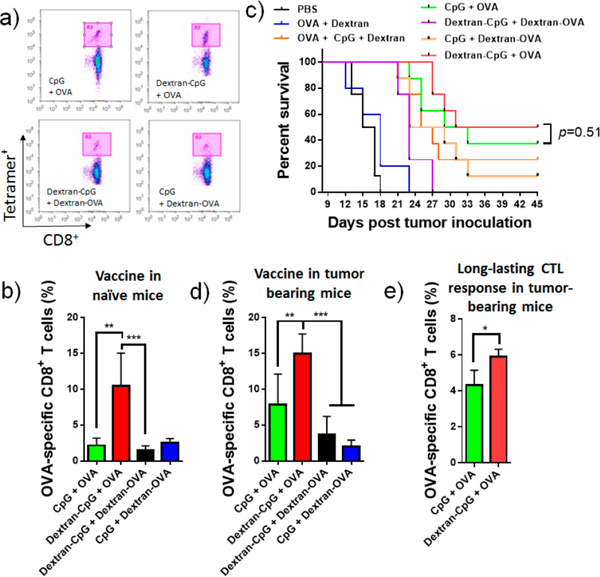
Figure 3.
Dextran-CpG conjugate, when combined with a soluble protein antigen, elicits robust expansion of antigen-specific CD8+ T-cells with therapeutic benefits, as compared to soluble formulations. (a,b) C57BL/6 mice were primed on day 0 and boosted on day 14 with Dextran-CpG and Dextran-OVA (10 μg OVA protein) or equivalent soluble protein/CpG vaccines. Six days post boost, mice were bled and analyzed for tetramer positive CD8+ T-cells in peripheral blood. (a) Representative flow cytometry dot plots of H2Kb/SIINFEKL tetramer staining of CD8+ cells. (b) Mean percentages of OVA-specific CD8+ T cells. (c,d) C57BL/6 mice (n = 8/group) were inoculated with 3 × 105 EG7 tumor cells s.c. in the flank and immunized with soluble or dextran-conjugated vaccines on days 6 (10 μg OVA, 1.24 nmol CpG) and 13 (20 μg OVA, 1.24 nmol CpG). Kaplan−Meier survival curves of eight mice per group are shown in (c). (d) Frequencies of OVA-specific CD8+ T cells in tumor bearing mice were determined 6 days post the final treatment. (e) On day 70 post tumor inoculation, tumor-free mice were bled and OVA-specific T cells in the blood were measured by tetramer staining. Data show the mean values ± SEM: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; n.s., not significant.