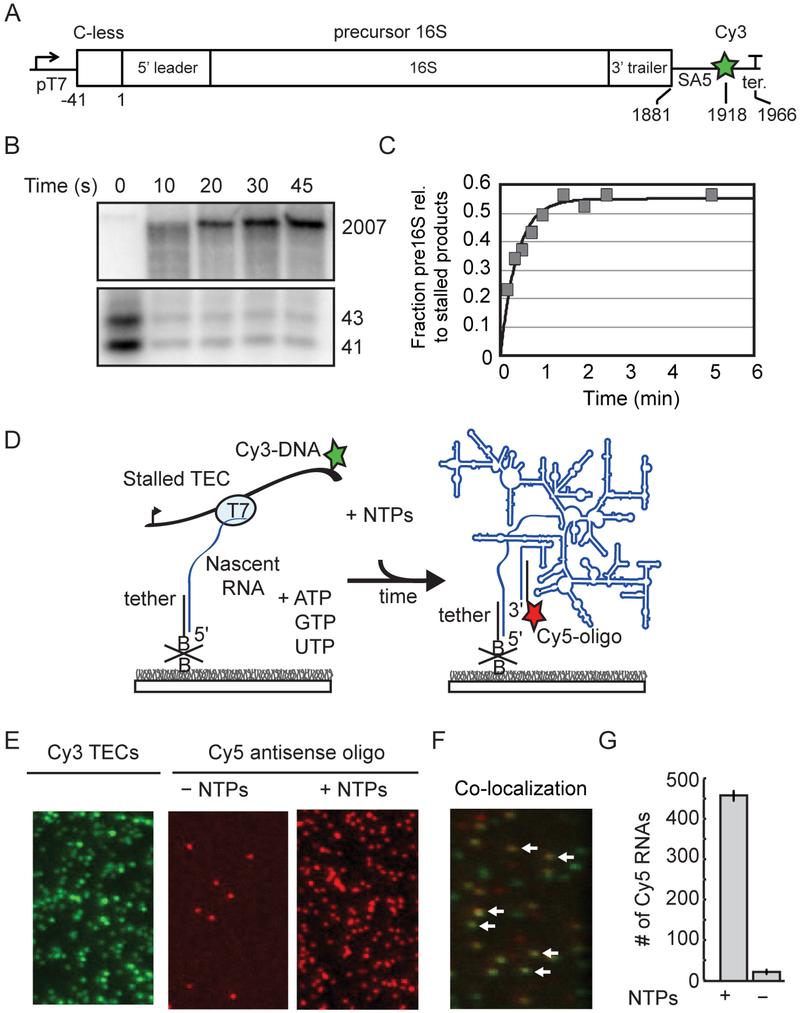
Figure 1.
Pre-rRNA transcription from single immobilized TECs. A) DNA template contains a T7 promoter and C-less cassette before the P2 transcription start site of the rrnB operon. See also Table S1. SA5 sequence is complementary to fluorescently labeled oligomer (SA5_aadU3; see Key Resources Table) used for labeling the transcribed RNA. Cy3 is attached to the template strand just upstream of the terminator. B) Single-round transcription assay demonstrating that full length pre-16S RNA can be generated from stalled TECs stalled in the absence of CTP. 32P-labeled products were resolved by 6% PAGE; top and bottom regions containing major bands are shown. Lanes were cropped from the same gel and are shown at the same contrast. C) Quantitation of gel in B. D) Stalled TECs (–CTP) are immobilized on passivated microscope slides through a biotinylated oligomer complementary to the 5′ end of the transcript, and then restarted with injection of NTPs to the slide chamber. RNA is detected using a Cy5-oligomer complementary to 3’ end of the transcript (SA5_aadU3). E) Representative microscope images showing that localization of Cy5-oligomer depends on NTP addition. Stalled Cy3-TECs imaged prior to NTP addition (left); Cy5-oligomer was added to the slide chamber in the absence (middle) or presence of NTPs (right) before imaging. F) Overlay of a different region illustrating colocalization between immobilized Cy3-labeled TECs and full-length RNA after NTPs and a Cy5-labeled oligomer (SA5_aadU3) were added to the slide chamber. White arrows highlight several instances of co-localization indicating that transcription has occurred at these sites. G) Quantification of 10 fields of view; mean ± s.d.