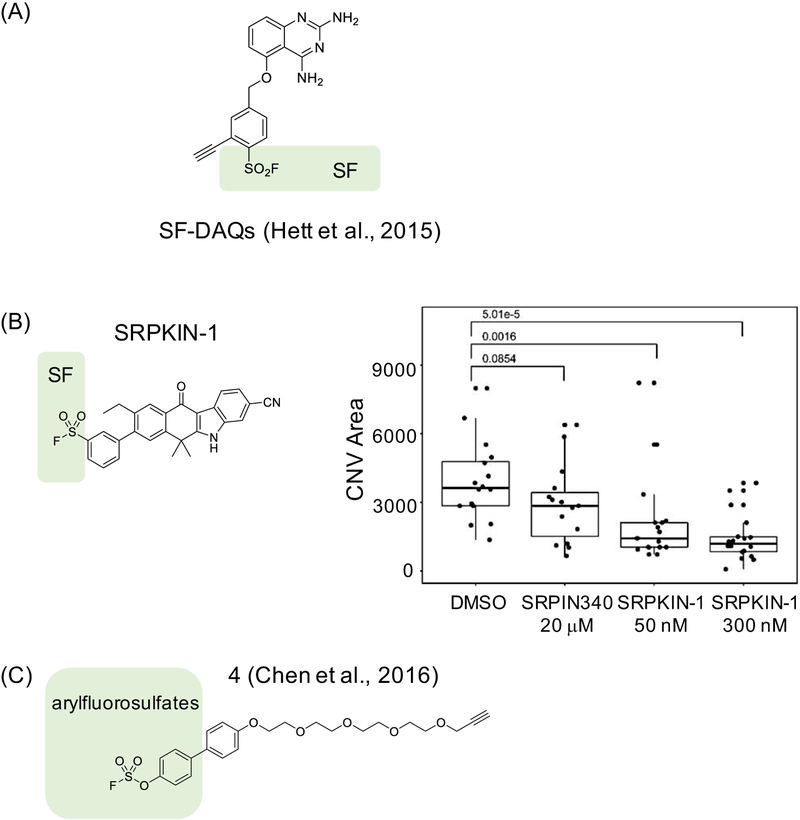
Figure 4. Examples of covalent ligands developed to target tyrosines.
(A) A series of sulfonyl fluoride (SF)-containing diaminoquinazolines (DAQs) compounds that irreversibly inhibit mRNA decapping scavenger protein DcpS by targeting Tyr113 (meta- and ortho-isomers) or Tyr143 (para-isomers) (Hett et al., 2015), (B) SRPKIN-1 is a Tyr-directed inhibitor with an SF warhead that forms a covalent bond with Tyr227 on SRPK1/2. Biological effects induced by SRPKIN-1 and a reversible control compound SRPIN-340 in a mouse model of wet age-related macular degeneration (wet AMD) display clear differences, supporting that covalent binding is critical for bioactivity. The experiment shown was conducted using laser-induced choroidal neovascularization (CNV) at concentrations of compounds as indicated, and the extent of the effect was quantified as CNV area – here inhibition of SRPK with SRPKIN-1 leads to changes in VEGF splicing resulting in production of anti-angiogenic VEGF-A165b isoform and decrease in new blood vessel formation as measured by the size of CNV area; and (C) arylfluorosulfate-containing probe 4 targeting Tyr134 on CRABP2 (Chen et al., 2016).