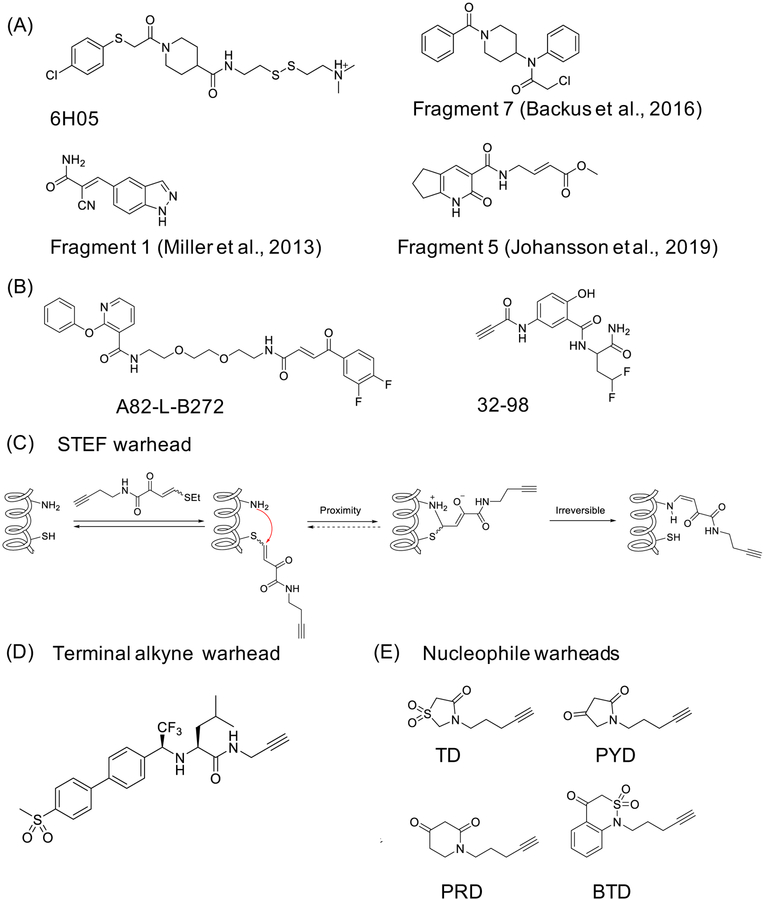
Figure 6. Selective covalent ligand discovery through library screening and warhead development.
(A) Fragment-based ligand discovery (FBLD) efforts led to discovery of the following fragments: 6H05 for KRASG12C (Ostrem et al., 2013), fragment 1 for MSK/RSK (Miller et al., 2013), fragment 7 for CASP8 and CASP10 (Backus et al., 2016), and fragment 5 for HOIP (Johansson et al., 2019); (B) example compounds developed using DNA-encoded chemical libraries that target JNK1 (Zimmermann et al., 2017), and ERBB2 (Zambaldo et al., 2016); (C) b-heteroatom substituted acrylamides warheads STEFs, and their mechanism (Hansen et al., 2019); (B) terminal alkyne as a warhead, here included into a potent cathepsin K (CatK) inhibitor, odanacatib (Mons et al., 2019); and (C) nucleophilic warheads that target sulfenic acid (Cys-SOH): benzo[c][1,2]thiazine (BTD), pyrrolidine-2,4-dione (PYD), piperidine-2,4-dione (PRD), and thiazolidine-4-one 1,1-dioxide (TD) (Gupta et al., 2017).