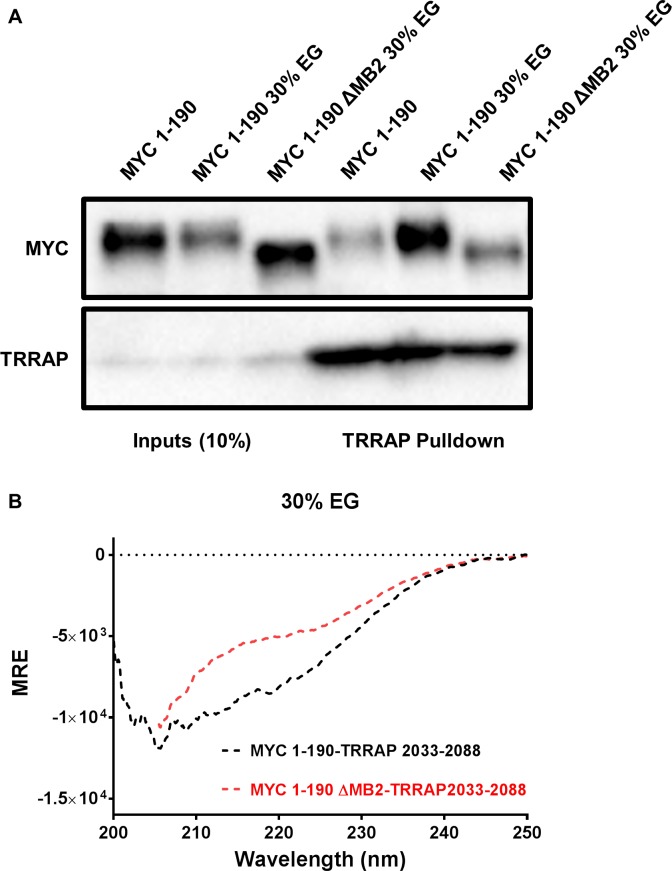
Fig 4. The effect of EG on MYC-TRRAP.
(A) Coomassie-stained SDS-PAGE of a pulldown of two 3C protease-cleavable fusion proteins. MYC 1-190-TRRAP 2033-2088-TS and MYC 1–190 ΔMB2-TRRAP2033-2088-TS incubated in either 1X PBS or 30% EG. After 3C cleavage of the linker, the TRRAP domain was pulled down with StrepTactin® beads and the EG was washed away with 1X PBS. MYC 1–190 showed enhanced binding to TRRAP 2033–2088 in the presence of 30% EG but not in PBS, when compared to MYC 1–190 ΔMB2 in 30% EG. (B) CD spectra of two MYC-TRRAP fusion proteins in 30% EG: MYC 1-190-TRRAP 2033–2088 in black and MYC 1–190 ΔMB2-TRRAP 2033–2088 in red. The effects of EG on the fusion protein containing MB2 are more profound and are indicative of a specific gain in α-helical character.