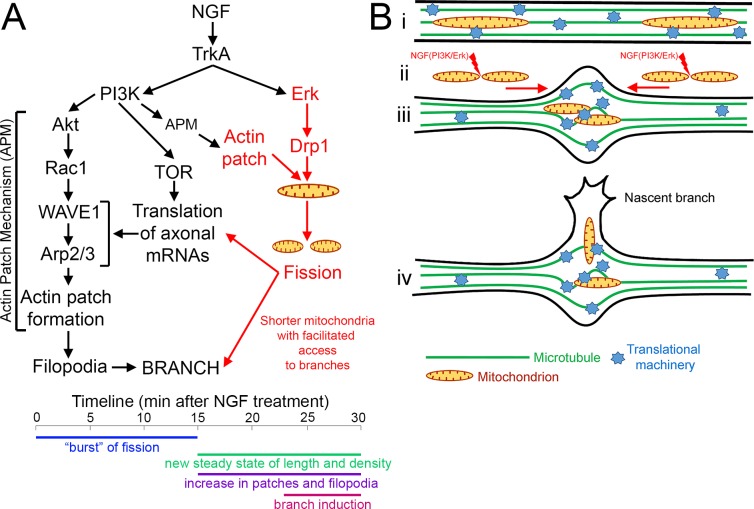
Figure 8. Summary and hypothetical mechanism for the role of fission in allowing mitochondria to target to sites of NGF-induced sensory axon collateral branching.
(A) Schematic summary of previously established components of the mechanism of NGF-induced axon branching (black text; modified from Spillane et al., 2012) and the role of NGF-induced mitochondria fission elucidated herein (red text). The formation of actin patches is dependent on a PI3K-Rac1-Arp2/3 based signaling and cytoskeletal mechanism (actin patch mechanism, APM). This mechanism driving formation of actin patches is dependent on PI3K-Akt signaling both in the context of NGF-signaling and in the absence of NGF (Ketschek and Gallo, 2010). NGF increases the rate of actin patch formation, in part, by driving the intra-axonal translation of relevant actin regulatory molecules (cortactin, Arp2, WAVE1; Spillane et al., 2012) required for patch formation. Herein, we show that NGF also induces the fission of axonal mitochondria during the first 10 min of signaling, prior to branching and the axonal translation-dependent increase in actin patch formation. The fission requires NGF-induced activation of Drp1 through Erk signaling. The fission also requires actin filaments that present as patches at the site of fission. Based on the requirement for actin filaments, PI3K-Akt signaling and the Arp2/3 complex for the formation of actin patches and fission, we suggest the role of PI3K in fission is mediated through the previously described APM also regulating the actin patch component of the fission machinery. The ensuing fission results in mitochondria with lengths that facilitate their targeting into nascent branches. The fission is also required for the NGF-induced and mitochondria-dependent intra-axonal translation of cortactin (Spillane et al., 2013). The hypothetical mechanism by which fission may contribute to axonal translation is presented in the panel B. The approximate time line of the events described above is presented below. The NGF-induced burst of fission occurs during the first 10–15 min of signaling, after which the new steady state of length and density is established along axons. The fission precedes the subsequent axonal translation dependent increase in the formation of actin patches and filopodia (Ketschek and Gallo, 2010; Spillane et al., 2012; Spillane et al., 2013). The emerge of new branches from the axon follows becoming evident by after 20 min of NGF treatment (Spillane et al., 2012). (B) Graphical representation of the hypothetical contribution of fission to the establishment of localized sites of intra-axonal protein synthesis during NGF-induced axon collateral branching. This hypothetical model is derived from the synthesis of from multiple prior studies showing the accumulation of organelles and translational machinery at sites of branching (Yu et al., 1994; Spillane et al., 2013; Courchet et al., 2013; Wong et al., 2017), the correlation of sites of branching with localized microtubule splaying (Dent and Kalil, 2001; Ketschek et al., 2015) and the observation that NGF induces microtubule splaying by 5 min post treatment (Ketschek et al., 2015), the time when mitochondria are undergoing fission and redistribution within the axon. (i) before NGF treatment mitochondria are longer, and translational machinery is undergoing transport and stalling throughout the axon in an unregulated manner. (ii) Signaling through PI3K and Erk is required for the fission of axonal mitochondria in response to NGF, and the fission correlates with transport mediated redistribution of mitochondria within the axon. (iii) as mitochondria undergo redistribution they encountered segments of the axon within which microtubules have undergone splaying apart and correlate with sites of potential branching. Through an unspecified mechanism transport cargoes undergo stalling in the axon segments where microtubules have splayed and accumulate resulting in the mitochondria and translational machinery. This converge then assists in the establishment of axon segments with high translation potential that correlate with sites of potential axon branching. (iv) Axon branches can then arise from these specialized domains through a multifaceted process involving the regulation of the actin cytoskeleton, microtubules, intra-axonal protein synthesis and directed transport into branches. The shorter length of mitochondria following fission is permissive for the entry of mitochondria into nascent branches.