Purpose:
To evaluate early-stage prostate cancer (PCa) radiotherapy treatment patterns and outcomes among Ghanaian men (GM) compared with US men (USM).
Materials and Methods:
This retrospective study consists of 987 National Comprehensive Cancer Network low risk, favorable intermediate risk, and unfavorable intermediate risk PCa patient subgroups; GM (173) and USM (814). Differences in baseline covariates and clinical characteristics between GM and USM were analyzed using χ2 and Mann-Whitney test while Cox Proportional Hazards model was used to assess freedom from biochemical failure differences between the study groups.
Results:
Median follow-up for this study was 40 months. GM were diagnosed at a younger median age (64 vs. 68 y, P<0.001) with heavier unfavorable intermediate risk disease burden (32.4% vs. 19.2%) compared with USM. Significant differences were identified in median external beam radiotherapy dose (72.4 vs. 78 Gy, P<0.001); brachytherapy utilization (49.7% vs. 80.6%, P<0.001) and androgen deprivation therapy for intermediate risk disease (48.4% vs. 21.0%, P<0.001) between GM and USM, respectively. GM with low risk and favorable intermediate risk PCa were at increased risk of biochemical recurrence compared with USM with adjusted hazard ratio: 5.15 (1.27 to 20.7), P=0.02 and 4.64 (1.20 to 17.92), P=0.02, respectively.
Conclusions:
Compared with USM, GM with low and favorable intermediate risk PCa may experience less durable disease control following standard treatment recommendations. Results suggest differences in radiation treatment and possible inherent differences between the 2 populations. This data will aid in developing research strategies to improve treatment outcomes in GM.
Key Words: low risk, intermediate risk, definitive radiation treatment, freedom from biochemical failure, Ghanaian men, US men
Prostate cancer (PCa) remains a disease of significant public health importance globally and exhibits regional variation in incidence and mortality patterns.1 It is the leading cancer diagnosis among men in Africa with higher mortality rates relative to western countries partly because of inadequate access to diagnostic and treatment facilities. Radiation treatment by external beam radiotherapy (EBRT) and brachytherapy remains an integral armor in the management of PCa. However, reviews of installed capacity for radiotherapy treatment in Africa continue to paint an ominous picture regarding access to quality care for patients requiring radiation treatment in low to middle-income countries which are projected to bear two thirds of new cancer burden globally by 2020.2,3 In most of these countries poor access leads to low radiotherapy utilization rates.4
A Ghanaian cancer center National Radiotherapy Oncology and Nuclear Medicine Centre, Korle Bu Teaching Hospital (NRONMC) in Accra, Ghana, has been offering 3-dimensional conformal external beam radiotherapy (3DCRT) via a telecobalt therapy unit (Co-60) and iodine (I125) brachytherapy services to PCa patients in the West African sub region for over a decade. The facility sees over 1200 new cancer patients annually with PCa accounting for 10% to 15% of this figure with an upward trend in numbers from a recent review.5 PCa brachytherapy outcomes from a Hospital in Ghana revealed disease control rates comparable to historical controls from high volume centers.6–9
The facility has recently begun offering EBRT with a modern linear accelerator unit. It is necessary to evaluate treatment outcomes in Co-60 era to consolidate gains made and identify potential avenues for improvement and research opportunities in this transition. This study is a cross-continental comparative study of National Comprehensive Cancer Network (NCCN) low and intermediate risk category PCa patients treated at NRONMC and a Moffitt Cancer Center and Research Institute (MMC), aimed at investigating curative radiation therapy outcomes and exploring determinants of these outcomes in low and intermediate risk PCa patients treated at the 2 centers with the goal of identifying avenues for technology exchange and further improvement in patient outcomes.
MATERIALS AND METHODS
Patient Selection
This study received Institutional Review Board (IRB) approval from NRONMC and MCC, utilizing the databases of NRONMC and Radiation Oncology Department of MCC covering the period from 2002 to 2016. Clinical records of 1084 patients treated at NRONMC and 1301 patients at US institution over this period were available for review.
To obviate the potential risk of confounding due to observed higher pretreatment prostate-specific antigen (PSA) among Ghanaian men (GM), only nonmetastatic NCCN low and intermediate risk patients were included in final analytical cohort (Gleason score≤7; PSA <20 ng/mL; and clinical stage T2C).10 Intermediate risk patients were further subdivided into favorable intermediate risk and unfavorable intermediate risk.11 In addition, patients who had received prior radical prostatectomy were also excluded. A total of 987 eligible patients from NRONMC (n=173) and US institution (n=814) were included in the final analysis.
Freedom from biochemical failure (FFBF) was the study end point. Biochemical failure was defined as nadir PSA+2 (according to the RTOG-ASTRO Phoenix Consensus Conference) and the period estimated from the date of treatment completion.12
RADIATION THERAPY
EBRT
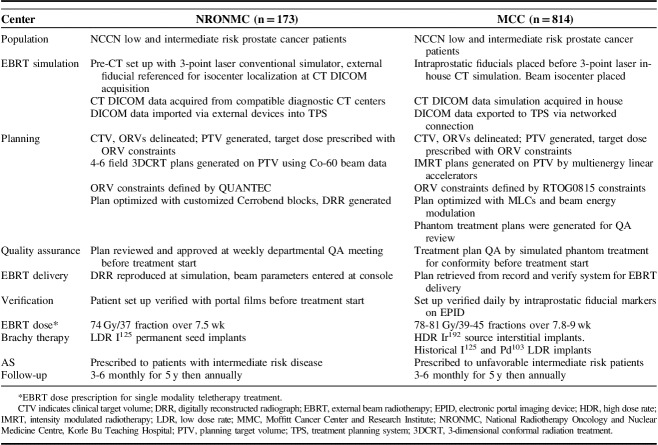
Treatment details at our study centers have been summarized in Table 1. Radiation treatment planning at NRONMC before 2008 was executed with 2-dimensional 4-field box technique generated by a conventional simulator. The treatment fields were defined by inferior sacroiliac joint; 1 cm superior to the ischial tuberosity; 1 to 2 cm lateral pelvic brim; mid pubic arch; and mid-point of S2/S3 interphase served as the superior, inferior, lateral, anterior and posterior borders, respectively, with customized Cerrobend blocking prescribed to 70 Gy in 35 fractions, 2 Gy/d, 5 days a week over 7 weeks.
TABLE 1.
Patient Population, Radiation Treatment Planning, and Delivery Summary
After 2008, all curative cases were planned with 3DCRT with a Prowess Panther version 4.6 treatment planning system (TPS). Patients were setup with the aid of the 3-point laser system of the conventional simulator in the absence of computerized tomography (CT) simulator. Fiducial marker reference points were marked on patients and recorded to enable marker placement before DICOM data acquisition at diagnostic CT scan centers. These CT scanners must have flat-top couch inserts and compatible DICOM format. The CT scanners were calibrated with a tissue characterization phantom (CIRS, USA) by Physicists from NRONMC to ensure tissue heterogeneity corrections by the TPS. The DICOM data were imported from external devices into the TPS for target volume delineation. The prostate was the clinical target volume (CTV) for low and favorable risk patients while the proximal 1 cm of seminal vesicles was included in the CTV for patients with unfavorable intermediate risk or >15% risk of seminal vesicle involvement on Partin Nomogram. The planning target volume (PTV) was generated by a 1 cm margin on the CTV in all directions except posteriorly which was expanded by 0.6 cm. Dose prescription to center was 74 Gy in 37 fractions over 7.5 weeks at 2 Gy per fraction respecting the Organ at Risk (ORV) constraints of the Quantitative Analysis of Normal Tissue Toxicity in the Clinic for bladder, rectum, and femoral heads (QUANTEC) which were enhanced using multiple treatment fields, wedges, and customized Cerrobend blocks. Treatment plans were subsequently evaluated with dose volume histograms, depicted in Figure 1 and QUANTEC dose constraints.13 Treatment field parameters were transferred onto patient with the aid of a conventional simulator and digitally reconstructed radiographs generated for planned treatment fields. Before treatment delivery, treatment plans were taken through pretreatment quality assurance review. Portal imaging of all treatment fields was performed before treatment and midway, reviewed and approved by the treating physician.
FIGURE 1.
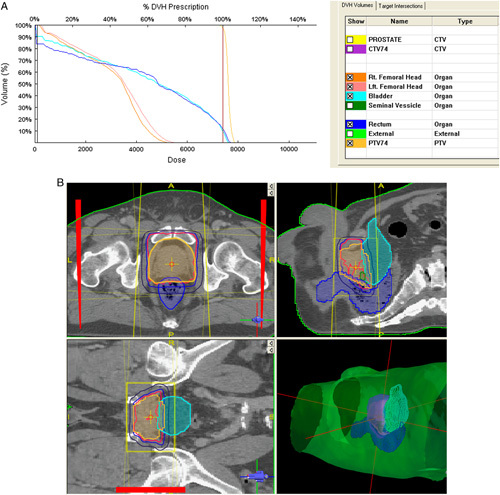
Three-dimensional conformal radiotherapy plan treated on Co-60 teletherapy unit at National Radiotherapy Oncology and Nuclear Medicine Centre, Korle Bu Teaching Hospital. CTV indicates clinical target volume; DVH, dose volume histograms; PTV, planning target volume.
EBRT at MCC was delivered by intensity modulated radiotherapy technique in >95% of cases using multienergy linear accelerators (Linacs) which employed motorized wedges and multileaf collimation to optimize PTV coverage while achieving ORVs objectives. Treatment planning CT DICOM data were acquired in-house and networked to the TPS where the CTV defined as at NRONMC was contoured with ORVs. A 7 mm margin PTV (5 mm posteriorly, inferiorly, and superiorly) generated on the CTV was prescribed 78 to 81 Gy in 39 to 45 fractions in 8 to 9 weeks at 1.8 to 2 Gy per fraction. Treatment plan quality assurance (or patient-specific dosimetry) was undertaken on treatment phantoms before treatment delivery and image guidance was employed for daily patient set-up. A record and verify system automatically recorded all delivered treatments per patient and verified this data against the planned treatment.
Brachytherapy
Template-based low-dose rate brachytherapy with I125 permanent seeds (Bard Medical Division, Covington, GA) planned in real-time intraoperatively and implanted transperineal under ultrasound guidance is the practice at NRONMC. Low and favorable intermediate risk patients received 160 Gy while higher risk patients received partial implant prostate boost of 110 Gy to the 90% isodose line pre-TG 43 formalism in combination with EBRT. Constraints were D30 <150% of prescribed dose for urethra and V100<1.3 cm3 for rectum.
Brachytherapy at US institution in the earlier years included Palladium (Pd103) and I125 low-dose rate seed implants (similar to NRONMC), as well as Iridium (Ir192) high-dose rate (HDR) brachytherapy. However, this practice has been supplanted by CT-planned HDR in the last decade. Transperineal HDR catheter placement guided by ultrasound and preoperative staging pelvic magnetic resonance imaging was performed in the operating room. Treatments were delivered in the HDR suite with monotherapy dose of 28 Gy in 2 fractions or boost dose of 23 Gy in 2 fractions spaced by 2 to 4 weeks. Single boost prescriptions of 15 Gy were less commonly utilized.
ANDROGEN DEPRIVATION THERAPY (ADT)
ADT for 4 to 6 months with either goserelin acetate (Zoladex) or leuprolide (Lupron) was prescribed with the start of radiotherapy based on patient’s disease risk profile and treatment modality. ADT was also prescribed for patients with large prostate volumes for cytoreduction purposes to render them eligible for brachytherapy.
FOLLOW-UP
Patients were reviewed at weekly clinic visits during their course of EBRT and within a week of brachytherapy procedure for treatment-related toxicity. Patients were seen with PSA reports at 3 to 6 months intervals upon treatment completion in the first 5 years and yearly thereafter with PSA reports.
Statistical Analysis
Patients’ baseline characteristics including demographic, clinical, and treatment information were summarily described. In addition, our study cohorts were stratified by treatment received and NCCN risk groups. The differences between the 2 populations were analyzed using methods of categorical analysis (USA and Ghana). χ2 test for categorical and Mann-Whitney test for numeric variables was used. In the outcome analysis a Cox proportional hazard model along with Kaplan-Meier analysis was used to estimate the risk of biochemical recurrence (FFBF) postradiotherapy between the US and Ghana cohort. Multivariable cox model stratified using NCCCN risk groups was adjusted for differences in radiotherapy and the use of hormonal therapy. Risk estimates using both unadjusted hazard ratio and adjusted hazard ratio (HR) along with 95% confidence interval (CI) were reported. Finally, all the variables used in the multivariate cox model were also assessed for their time-varying effect to ensure that the proportionality assumption holds true. None of the variables used in the model showed a deviation from the proportionality assumption. Two-sided α value <0.05 was considered as statistically significant. All the analysis was completed using SAS 9.4.
RESULTS
Patient and Disease Characteristics
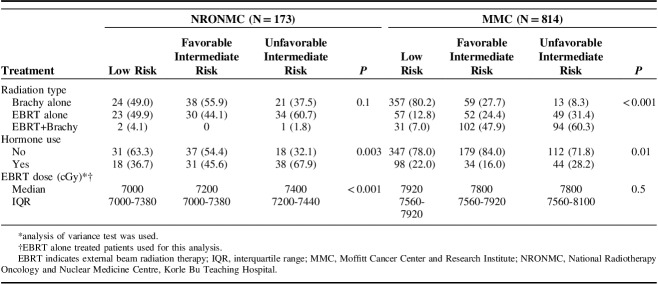
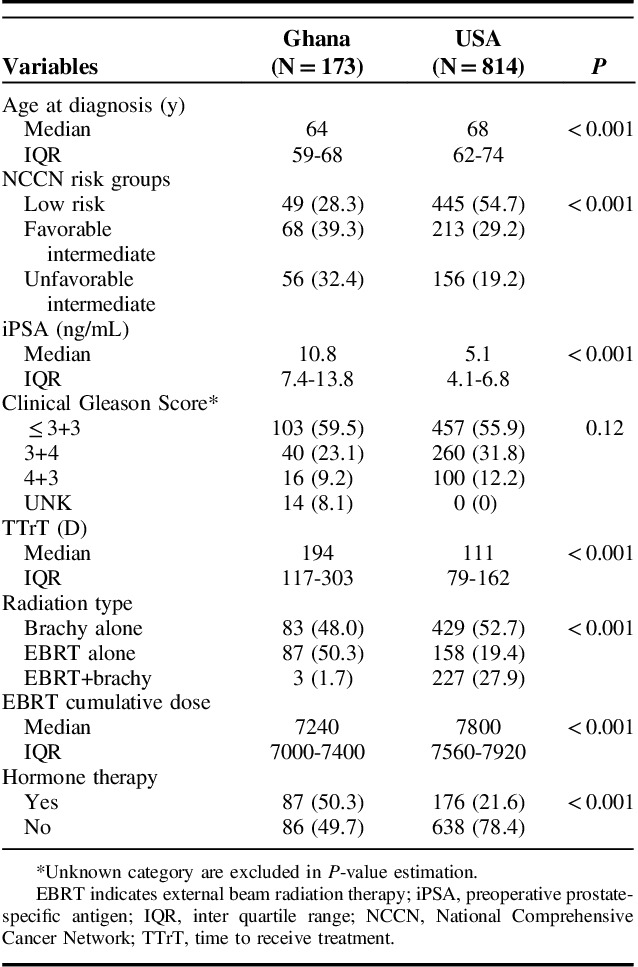
This retrospective cross-continental comparative paper evaluated disease presentation and treatment-related factors among 987 NCCN low and intermediate-risk PCa patients pooled from the largest referral facility in Ghana (GM, n=173) which serves the entire West African subregion and a US institution (US men [USM], n=814), an NCI-designated comprehensive cancer center and covered a 15-year period. The combined median follow-up for GM of 40 months (interquartile range: 22 to 80) and USM of 36 months (interquartile range: 18 to 58) were comparable. GM were diagnosed at a younger median age (64 vs. 68 y, P<0.001); presented more frequently with unfavorable intermediate risk disease (32.4% vs. 19.2%) and higher PSA (10.8 mg/dL vs. 5.1 mg/dL) while most USM presented with low risk disease (54.7% vs. 28.3%) as shown in Table 2. Also, GM experienced longer intervals from diagnosis to initiation of radiation treatment (median: 194 d vs. 111 d, P<0.001) (Table 2).
TABLE 2.
Baseline Patient Characteristics at Ghana and US Institutions
Treatment and Outcomes
Compared with USM, GM received 5.6 Gy lower median EBRT dose and were less likely to receive brachytherapy as EBRT boost or monotherapy (49.7% vs. 80.7%, P<0.001) as shown in Tables 2 and 3. Differences in brachytherapy utilization were more marked among unfavorable intermediate-risk patients with only 1.8% GM receiving this treatment compared with 60.3% of USM in this risk category (Table 3). ADT utilization was more than twice as likely among both favorable intermediate risk and unfavorable intermediate risk GM compared with USM (45.6% vs. 16%, and 67.9% vs. 28.2%, respectively) (Table 3).
TABLE 3.
Comparison of Treatment at Ghana and US Institutions Stratified by NCCN Risk Category
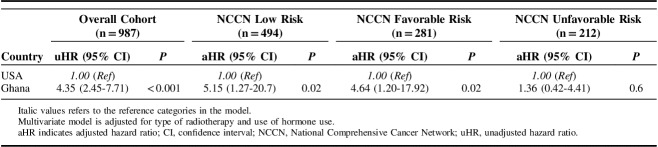
The NCCN risk stratified unadjusted rates of biochemical recurrence in Kaplan-Meier analysis showed less durable FFBF among GM compared with USM across all risk categories (Fig. 2). However, in the treatment adjusted and NCCN risk stratified multivariable cox model (Table 4), risk of biochemical recurrence was only significantly higher among low (HR=5.15; 95% CI, 1.27-20.7; P=0.02) and favorable intermediate risk (HR=4.64; 95% CI, 1.20-17.92; P=0.02) GM compared with USM. No difference in biochemical control was observed among unfavorable intermediate risk patients from our study cohorts (HR=1.36; 95% CI; 1.36-4.41; P=0.6).
FIGURE 2.
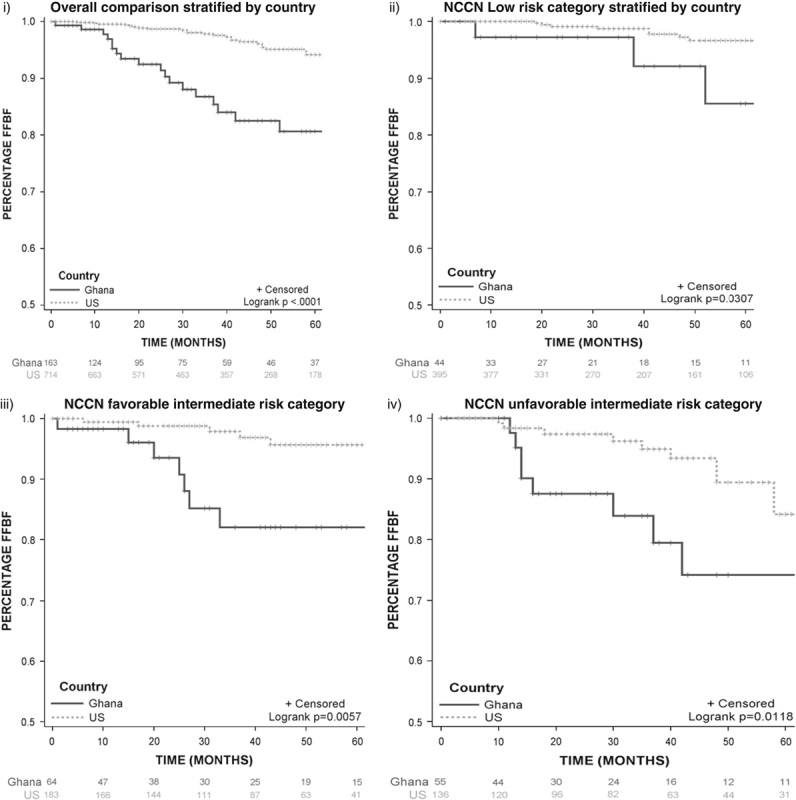
Kaplan-Meier graphs estimating freedom from biochemical failure for Ghana and Moffitt Cancer Center and Research Institute. FFBF indicates Freedom from biochemical failure; NCCN, National Comprehensive Cancer Network.
TABLE 4.
Risk of Biochemical Recurrence Among US and Ghanaian Men by NCCN Risk Category
DISCUSSION
Modern radiation dose delivery via 3DCRT and intensity modulated radiotherapy with image-guided conformal treatment techniques and use of brachytherapy have revolutionized PCa treatment. EBRT dose escalation delivered with highly innovative and sophisticated therapy units with brachytherapy result in better biochemical control in low and intermediate risk groups.14,15 Setbacks to the universal adoption of this technology, however, are the need for stable and constant electricity supply; high cost of initial investment and maintenance; and personnel in under-resourced environments.16,17 The disease control benefit and toxicity profile of these technologies have not been adequately examined when discussing the global deficit of radiotherapy facilities particularly in low to middle-income countries.3,16 This has become more imperative as patients increasingly present with the curable disease. The NRONMC has until recently delivered EBRT utilizing a Co-60 unit without multileaf collimation and onboard portal imaging. All PCa patients with localized disease who opt for nonsurgical treatment were treated with 3DCRT techniques. Limitations of this unit include averagely higher integral doses to normal tissues and a larger penumbra compared with linear accelerators which make it difficult to achieve precise organs-at-risk constraints at higher radiation doses with conformal techniques especially in patients with wider separation. Highly complex field arrangements in the absence of multileaf collimators and on board imaging are not practical at present but anticipated in the near future with the installation of a 6 MV linear accelerator.
Patient and Disease Characteristics
This study specifically focused on patients with low risk of harboring metastatic disease to minimize its likelihood of confounding the observed outcome. A recent NRONMC PCa study spanning 15 years indicates that only 10% of PCa referrals to the center were eligible for this study accounting partly for the small number of patients from NRONMC.18 Patients treated at NRONMC presented at a younger age compared with the US cohort and had a proportionately higher burden of unfavorable intermediate risk. This is contrary to reports associating older age with advanced PCa from registries such as the Cancer of the Prostate Strategic Urologic Endeavor (CaPSURE) as Gleason Scores generally increase with age.19,20 Therefore, it is possible that the overall difference in age at diagnosis and the presentation with the less favorable disease at a younger age in the Ghana cohort may be surrogates for differences in disease biology.
Radiotherapy Delivery
The pattern of radiotherapy treatment was significantly different between the 2 cohorts in this analysis with nearly 2-fold brachytherapy utilization rate among USM as GM. This difference was more evident among unfavorable intermediate risk patients with <5% of GM receiving brachytherapy boost. This makes it difficult to evaluate the benefit of incorporating brachytherapy in the treatment of this population of patients in our study. Furthermore, a higher median EBRT dose was achievable among USM as a result of the use of multienergy linacs in treatment planning and delivery under daily image guidance. Both of these factors may have accounted for better FFBF among USM consistent with the established benefit of radiation dose escalation in disease control.14,15
Use of intraprostatic fiducial markers for prostate localization during EBRT is the practice at US institution. A study from Mayo clinic reported the prostate movement of >1 cm in some instances between treatment fractions.21,22 Fiducial-based prostate localization is comparable to using cone-beam CT for prostate localization and this may be explored as an avenue to dose escalate in Ghana.23–25
Androgen Suppression
Androgen suppression usage was higher among GM and was generally prescribed to intermediate risk patients who received EBRT alone to make up for the low dose received. Some low risk patients also received ADT either before referral to the NRONMC or, on a few occasions, for prostate volume reduction before brachytherapy. Two studies from the United States have reported improved FFBF outcomes among intermediate risk patients receiving 4 to 6 months ADT concomitant with EBRT but the prescribed radiation doses of 66.6 to 70 Gy in these studies would be considered suboptimal today and may explain to some degree the FFBF benefit seen with androgen suppression.26,27 Reported dose-escalated studies have generally omitted or reduced the duration of androgen suppression even among patients with high-risk disease.14,28,29 As a result, the role of androgen suppression in this setting remains unresolved but this may be clarified by on-going trials.30
Treatment Outcome
The estimated FFBF was less favorable among low and favorable intermediate risk but not unfavorable intermediate risk patients treated at NRONMC compared with US institution. The overall biochemical control achieved at the NRONMC, however, compares favorably to a similarly planned and dose-treated cohort which included a sizeable proportion of high risk patients.31 It is plausible that differences in radiation treatment delivery may have contributed to the observed differences in FFBF, however, adjusting for this in the Cox Proportional Hazards model failed to account fully for this difference. This finding suggests potential inherent differences in our study population contributing to this observation.
Efforts at Improving Treatment Outcomes
The NRONMC has installed a new 6 MV linear accelerator capable of arc therapy which will operate on a record and verify platform. Efforts are also underway to introduce image guidance for set-up verification to enable escalated EBRT delivery. In addition, on-going genomic-based clinical studies in collaboration with MMC are anticipated to provide some clarity on the biology of PCa in GM. These interventions are anticipated to significantly enhance treatment outcomes in our population and will be the subject of future reports.
Limitations
Limitations associated with this study include its retrospective design; differences in the socioeconomic status of our patient populations; variations in patterns of care as well as utilization of imaging in diagnosis, staging and treatment planning and delivery at our study institutions.
Our study remains extremely relevant as it provides valuable information on the uniqueness of disease and treatment characteristics of a population largely absent in the PCa literature situated within the context of treatment outcome attainable with current standard-of-care and documents adaptation of low-resource technology to improve care.
CONCLUSIONS
We highlight likely differences in risk profile and biochemical control among early-stage prostate patients undergoing radiation treatment from an African population and a predominantly Caucasian American population. Factors inherent in radiotherapy modality, dose and delivery as well as inherent differences in our study populations possibly account for this variation and need to be addressed to improve treatment outcomes.
Footnotes
Work was performed at the National Center for Radiotherapy, Oncology and Nuclear Medicine, Korle Bu Teaching Hospital, Accra, Ghana, West Africa and H. Lee Moffitt Cancer Center, Tampa, FL.
Supported by grant awards to K.Y. from the Prostate Cancer Foundation and P20-CA233255. F.A.A. was supported by an Audrey Myer Mars International Fellowship in Clinical Oncology, SPAMM 17-218-01, from the American Cancer Society.
The authors declare no conflicts of interest.
REFERENCES
- 1.McGuire S. World cancer report 2014. Geneva, Switzerland: World Health Organization, international agency for research on cancer, WHO Press, 2015. Adv Nutr. 2016;7:418–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datta NR, Samiei M, Bodis S. Radiation therapy infrastructure and human resources in low- and middle-income countries: present status and projections for 2020. Int J Radiat Oncol Biol Phys. 2014;89:448–457. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Wahab M, Bourque J-M, Pynda Y, et al. Status of radiotherapy resources in Africa: an International Atomic Energy Agency analysis. Lancet Oncol. 2013;14:e168–e175. [DOI] [PubMed] [Google Scholar]
- 4.Rosenblatt E, Fidarova E, Zubizarreta EH, et al. Radiotherapy utilization in developing countries: an IAEA study. Radiother Oncol. 2018;128:400–405. [DOI] [PubMed] [Google Scholar]
- 5.Calys-Tagoe BN, Yarney J, Kenu E, et al. Profile of cancer patients’ seen at Korle Bu teaching hospital in Ghana (a cancer registry review). BMC Res Notes. 2014;7:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mensah JE, Yarney J, Vanderpuye V, et al. Prostate brachytherapy in Ghana: our initial experience. J Contemp Brachytherapy. 2016;8:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yorozu A, Kuroiwa N, Takahashi A, et al. Permanent prostate brachytherapy with or without supplemental external beam radiotherapy as practiced in Japan: outcomes of 1300 patients. Brachytherapy. 2015;14:111–117. [DOI] [PubMed] [Google Scholar]
- 8.Stone NN, Stock RG. 15-year cause specific and all-cause survival following brachytherapy for prostate cancer: negative impact of long-term hormonal therapy. J Urol. 2014;192:754–759. [DOI] [PubMed] [Google Scholar]
- 9.Kosj Y, Beecham K, Hegarty SE, et al. Early results of prostate cancer radiation therapy: an analysis with emphasis on research strategies to improve treatment delivery and outcomes. BMC cancer. 2013:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8:145. [DOI] [PubMed] [Google Scholar]
- 11.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. [DOI] [PubMed] [Google Scholar]
- 12.Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. [DOI] [PubMed] [Google Scholar]
- 13.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28:1106–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong WW, Vora SA, Schild SE, et al. Radiation dose escalation for localized prostate cancer: intensity-modulated radiotherapy versus permanent transperineal brachytherapy. Cancer. 2009;115:5596–5606. [DOI] [PubMed] [Google Scholar]
- 16.Page BR, Hudson AD, Brown DW, et al. Cobalt, linac, or other: what is the best solution for radiation therapy in developing countries? Int J Radiat Oncol Biol Phys. 2014;89:476–480. [DOI] [PubMed] [Google Scholar]
- 17.Healy B, van der Merwe D, Christaki K, et al. Cobalt-60 machines and medical linear accelerators: competing technologies for external beam radiotherapy. Clin Oncol (R Coll Radiol). 2017;29:110–115. [DOI] [PubMed] [Google Scholar]
- 18.Vanderpuye V, Venkat PS, Fink AK, et al. Contemporary radiation treatment of prostate cancer in Africa: a ghanaian experience. J glob oncol. 2018;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konety BR, Cowan JE, Carroll PR. Patterns of primary and secondary therapy for prostate cancer in elderly men: analysis of data from CaPSURE®. J Urol. 2008;179:1797–1803. [DOI] [PubMed] [Google Scholar]
- 21.Schallenkamp JM, Herman MG, Kruse JJ, et al. Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int J Radiat Oncol Biol Phys. 2005;63:800–811. [DOI] [PubMed] [Google Scholar]
- 22.Keall PJ, Beckham WA, Booth JT, et al. A method to predict the effect of organ motion and set-up variations on treatment plans. Australas Phys Eng Sci Med. 1999;22:48–52. [PubMed] [Google Scholar]
- 23.Kupelian PA, Willoughby TR, Meeks SL, et al. Intraprostatic fiducials for localization of the prostate gland: monitoring intermarker distances during radiation therapy to test for marker stability. Int J Radiat Oncol Biol Phys. 2005;62:1291–1296. [DOI] [PubMed] [Google Scholar]
- 24.Moseley DJ, White EA, Wiltshire KL, et al. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line image-guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys. 2007;67:942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langen K, Jones D. Organ motion and its management. Int J Radiat Oncol Biol Phys. 2001;50:265–278. [DOI] [PubMed] [Google Scholar]
- 26.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. [DOI] [PubMed] [Google Scholar]
- 27.D’amico AV, Manola J, Loffredo M, et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–827. [DOI] [PubMed] [Google Scholar]
- 28.Beckendorf V, Guerif S, Le Prisé E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80:1056–1063. [DOI] [PubMed] [Google Scholar]
- 29.Morris WJ, Tyldesley S, Pai HH, et al. ASCENDE-RT*: a multicenter, randomized trial of dose-escalated external beam radiation therapy (EBRT-B) versus low-dose-rate brachytherapy (LDR-B) for men with unfavorable-risk localized prostate cancer. Am Soc Clin Oncol. 2015;98:275–285. [Google Scholar]
- 30.Martinez A. RTOG 0815: a phase III prospective randomized trial of dose-escalated radiotherapy with or without short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer. Prostate Cancer. 2012. [Google Scholar]
- 31.Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. [DOI] [PubMed] [Google Scholar]