Figure 6.
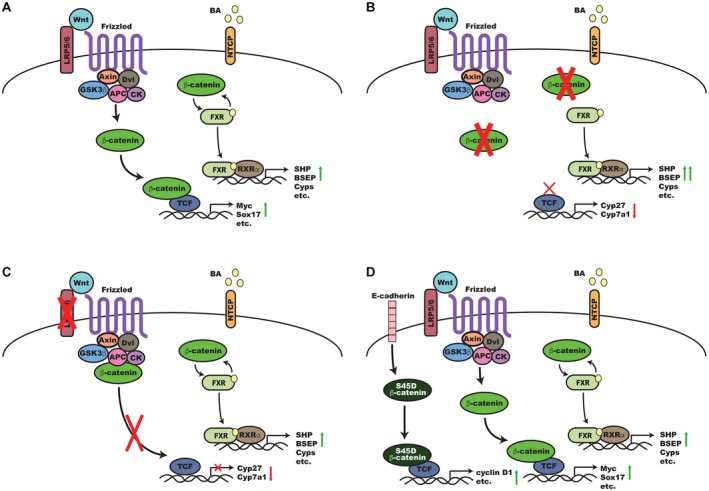
A proposed model for the regulation of β‐catenin and FXR activity during cholestasis. (A) In a normal liver after BDL, Wnts, such as Wnt7A, bind to coreceptors Frizzled and LRP5/6 KO, which triggers recruitment of Dvl and the β‐catenin destruction complex (Axin, APC, CK, andGSK3β) to the membrane. This results in hypophosphorylation of β‐catenin and its translocation to the nucleus where it binds to TCF to induce expression of target genes, such as Sox17 and Myc. Simultaneously, BAs accumulate and are taken up by NTCP; BAs bind to and activate FXR, which transiently dissociates from the inhibitory β‐catenin complex and, in partnership with RXRα, induces expression of target genes, such as BSEP and SHP. (B) In the absence of β‐catenin, transcriptional targets, such as Cyp27, are suppressed, while increased basal hepatic BAs also lead to compensatory suppression of Cyp7a1. Simultaneously, loss of the inhibitory β‐catenin/FXR complex results in increased activation of FXR, increased expression of downstream targets, such as SHP and BSEP, and optimal reduction and alteration of the hepatic BA pool during cholestasis. (C) In the absence of Wnt coreceptor LRP5/6, β‐catenin target genes are suppressed, but the continued presence of β‐catenin represses FXR and prevents optimal activation. (D) Mice overexpressing β‐catenin have similar kinetics of β‐catenin and FXR activation as normal mice except that S45D β‐catenin, which is usually sequestered at the hepatocyte membrane, is activated and turns on expression of additional β‐catenin targets, such as cyclin D1, that are involved in hepatocyte proliferation. Abbreviations: APC, adenomatous polyposis coli; Dvl, Dishevelled; GSK3, glycogen synthase kinase 3.
