Figure 6.
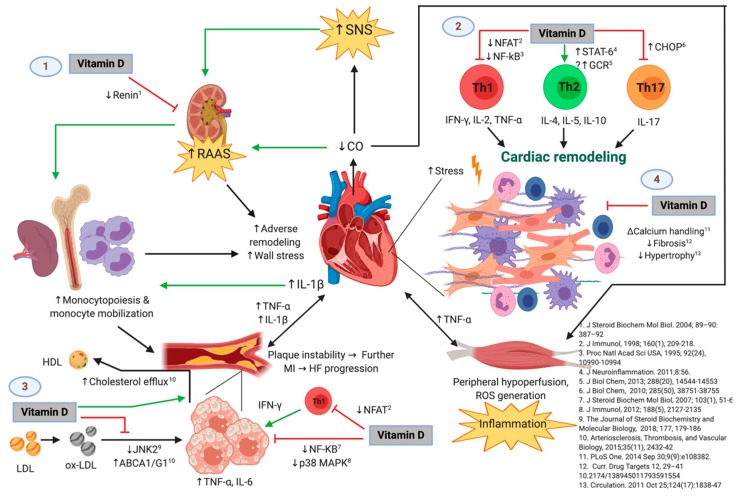
The Possible Immunomodulatory Role of Vitamin D in the Context of Heart Failure (HF) Progression. Green and black lines with arrowheads represent a direct stimulation and a positive correlation, respectively. Red lines ending in perpendicular bars indicate inhibition. (↑) represent an increase in expression, concentration, or activity of the parameter or mechanism. (↓) indicate a decrease in expression, concentration, or activity of the parameter of mechanism. HF is initiated and maintained by chronic maladaptive activation of neurohumoral networks that result from decreased cardiac output (e.g., the sympathetic nervous system [SNS] and the renin-angiotensin-aldosterone system [RAAS]), innate and adaptive immune mechanisms triggered by myocardial damage and stress, and systemic inflammation that results from hypoperfusion of peripheral organs [20,21]. Vitamin D may play a protective role in the progression of HF by interfering with several deleterious pathways [25], which include but are not limited to: (1) inhibiting the release of renin [44], which leads to attenuated RAAS-mediated adverse cardiac remodeling and release of monocytes from bone marrow and lymphoid tissue (an event that is also favored by IL-1β and perhaps other inflammatory cytokines from damaged myocardium) [21]. Monocytes are known to infiltrate damaged myocardium [45] and atheromatous plaques [35], where they exacerbate the local inflammatory response and promote further adverse ventricular remodeling as well as growth and instability of atherosclerotic plaques, respectively. Hence, vitamin D may indirectly attenuate monocyte/macrophage-mediated myocardial damage. (2) Vitamin D may also favor an anti-inflammatory T-helper lymphocyte phenotype by decreasing Th1 [46,47,48] and Th17 [9,23] cytokine production and favoring the Th2 phenotype [49], which would theoretically promote adequate remodeling and decreased fibrosis, and reduce cardiomyocyte hypertrophy and dysfunction. (3) Vitamin D could also slow the progression of atherosclerotic plaques by inhibiting the proinflammatory activation of foam cells (macrophages that have phagocytosed ox-LDL particles) through diminished ox-LDL capture [38], increased cholesterol efflux [37], and decreased activation of transcription factors involved in the expression of proinflammatory cytokines such as TNF-α and IL-6 [22,50]. Like the mechanism described above, vitamin D may also act on T-lymphocytes present in atherosclerotic plaques, decreasing IFN-γ production and hence proinflammatory polarization of macrophages/foam cells. This is of relevance, as growth of atherosclerotic plaques results in plaque instability and further myocardial infarction, with consequent exacerbation of the pathogenic loop that characterizes HF. (4) Vitamin D has also been demonstrated to exert direct effects on the cardiomyocyte and interstitium, including modulation of intracellular calcium handling [26], expression of procollagen-1 [27], and hypertrophic calcineurin/NFAT signaling [28]. Abbreviations: HF = heart failure; MI = myocardial infarction; ABCA1/G1 = ATP-binding cassette transporter types A1 and G1; CHOP = C/EBP homologous protein; CO = cardiac output; GCR = glucocorticoid receptor; IFN = interferon; IL = interleukin; JNK2 = c-Jun N-terminal kinase 2; LDL = low-density lipoprotein; HDL = high-density lipoprotein; NFAT = nuclear factor of activated T-cells; NF-κB = nuclear factor kappa-B; ox-LDL = oxidized low-density lipoprotein; p38 MAPK = p38 mitogen-activated protein kinases; RAAS = renin-angiotensin-aldosterone system; SNS = sympathetic nervous system; STAT6 = signal transducer and activator of transcription 6; Th = T-helper; TNF = tumor necrosis factor-alpha. Original image created with BioRender® (BioRender, Toronto, ON, Canada; website URL: https://biorender.com/; accessed on 9 October 2019).