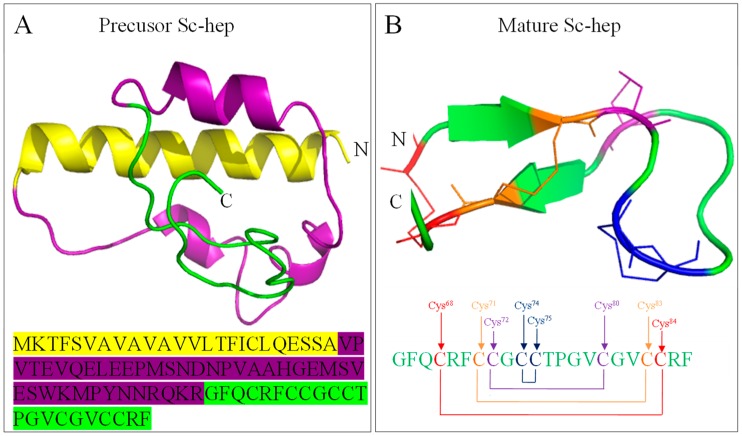
Figure A1.
Structure prediction of the Sc-hep protein. (A): Protein structure of the precursor Sc-hep. The yellow region is the signal peptide with an α-helix structure. The purple region is the propeptide with a random coil structure. The green region is the mature peptide with a β-sheet structure. (B): Structure of the mature Sc-hep protein. Four disulfide bonds are formed by eight cysteine residues to stabilize the whole fold of mature Sc-hep. The connection mode is distinguished by different colors. The red, orange, purple, and blue regions represent Cys68–Cys84, Cys71–Cys82, Cys72–Cys80, and Cys74–Cys75, respectively.