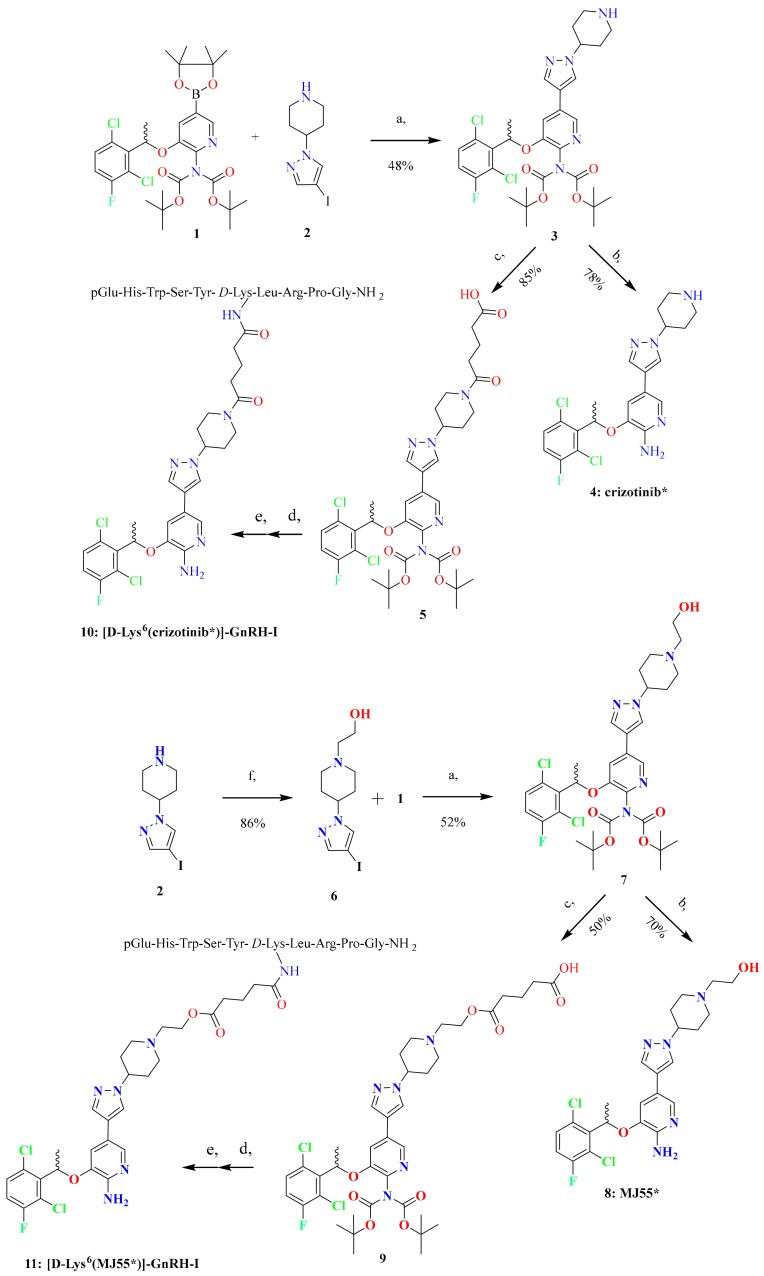
Figure 2.
Synthesis of crizotinib*, [d-Lys6(crizotinib*)]–GnRH-I, MJ55*, and [d-Lys6(MJ55*)]–GnRH-I. (a) 2 M Cs2CO3 in H2O, Pd(dppf)Cl2*, dimethyl sulfoxide, 70 °C, 3 h; (b) trifluoroacetic acid/water (9:1 ratio) 25 °C, 2 h; (c) glutaric anhydride, triethylamine, CH2Cl2, 25 °C, 2 h; (d) COMU**, 4-methylmorpholine, N,N-dimethylformamide, 25 °C, 2 h; (e) trifluoroacetic acid /phenol/water/triisopropyl silane (88:5:5:2 ratio) 25 °C, 2 h; (f) K2CO3, bromoethanol, tetrahydrofuran, reflux, 24 h. *Pd(dppf)Cl2—[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II). **COMU—(1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate.