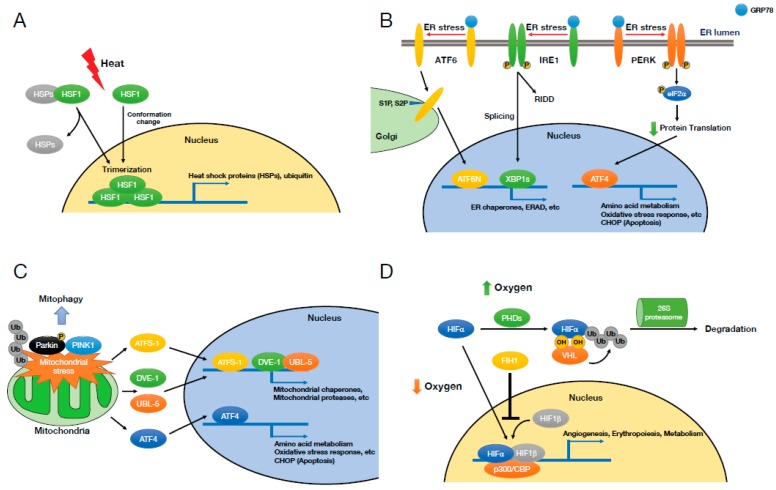
Figure 2.
Cellular stresses and signaling responses. (A) Heat shock stress and heat shock response. The stressors such as heat lead to releasing of heat shock proteins (HSPs) from heat shock factor 1 (HSF1) or directly changing the conformation of HSF1 resulting in its trimerization, nuclear translocation, and target gene transcription. (B) Endoplasmic reticulum (ER) stress and unfolded protein response (UPR). The accumulation of unfolded or misfolded proteins activates three ER transmembrane proteins—activating transcription factor-6 (ATF6), inositol requiring protein-1 (IRE1), and protein kinase RNA-like ER kinase (PERK). ATF6 and IRE1 generate the functional transcription factors, ATF6N and spliced form of X-box binding protein 1 (XBP1s), which translocate to the nucleus and transcribe their target genes, whereas PERK suppresses protein translation and thus reduces protein load into the ER. (C) Mitochondrial stress and mitochondrial unfolded protein response (UPRmt). Mitochondrial stress activates several transcription factors, activating transcription factor associated with stress-1 (ATFS-1) and defective proventriculus (Drosophila) homolog-1/ubiquitin-like 5 (DVE-1/UBL-5) (Caenorhabditis elegans) and ATF4 (mammals), which promote their target gene expression to restore mitochondrial homeostasis. Mitochondrial stress also triggers autophagy (mitophagy) via Parkin and Pink1. (D) Hypoxia and hypoxia-induced factor. Under normoxia, hypoxia-inducible factor α (HIFα) is hydroxylated on proline by prolyl hydroxylase domain enzymes (PHDs) or on asparagine by factor inhibiting HIF1 (FIH1), and the activity of HIFα is suppressed by its von Hippel–Lindau (VHL)-mediated ubiquitylation and degradation or its loss of the interaction with p300/CREB-binding protein (CBP).