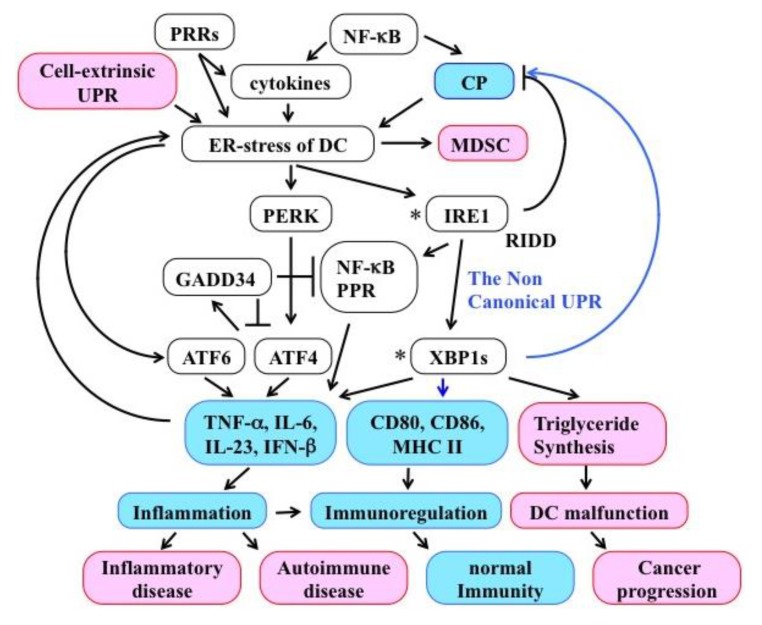
Figure 3.
The crosstalk between CP, UPR, and inflammation. Pathogen-related stimuli, such as pattern recognition receptor (PRR) ligation, cytokines, and cell-extrinsic UPR can induce ER stress in Dendritic cells (DCs). Accumulation of unfolded proteins for CP also contributes to trigger/sustain ER stress in DCs. Although ER stress activates the UPR to restore ER homeostasis, the UPR itself accelerates the transcription of several inflammatory cytokines both directly, via UPR-related transcription factors, and indirectly, through the activation of different signaling cascades (e.g., NF-κB, PRRs). When the UPR of DCs is over-activated, the immunoregulatory functions of DCs are dismissed and this may result in chronic inflammation and ultimately cancer. However, when the UPR is over-repressed, CP efficiency is impaired. Thus, the non-canonical UPR of DCs has to cope with opposite requirements in order to maintain both ER homeostasis and adequate CP/immune-modulatory functions. The precise molecular mechanisms to evade UPR over-activation are, however, not known, except for the established suppressive function of GADD33. Black arrows indicate activation, T-arrows indicate suppression, blue arrows indicate pathways involved in the non-canonical UPR. The asterisks of IRE1 and XBPs indicate the risk factor for inflammatory disease among the UPR-related molecules.