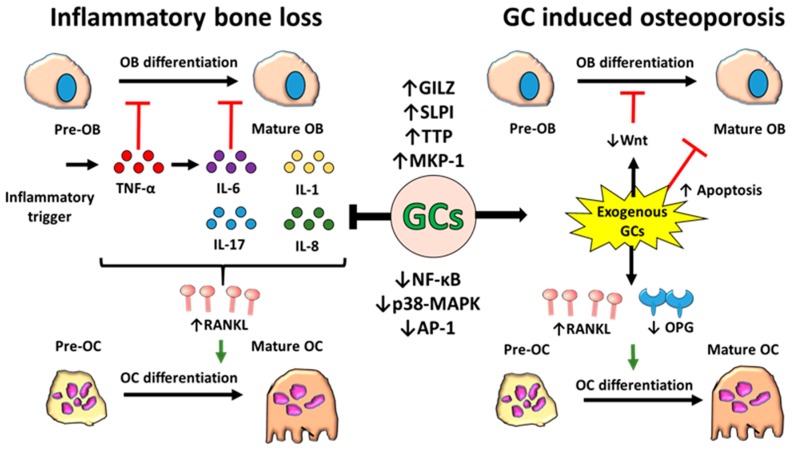
Figure 2.
Schematic representation of the effects of inflammation and glucocorticoids (GCs) on bone remodelling. During inflammation, elevated levels of pro-inflammatory cytokines, such as TNF-α and IL-6, inhibit the differentiation of bone forming osteoblasts from their precursors. These cytokines, along with other pro-inflammatory mediators including IL-1, IL-17 and IL-8, also upregulate the expression of receptor activator of nuclear factor kappa-Β ligand (RANKL), which binds to receptor activator of nuclear factor kappa-Β (RANK) on pre-osteoclasts and triggers their differentiation into mature bone resorbing osteoclasts. Overall, bone formation is decreased while bone resorption is increased, leading to a net loss of bone. Although GCs suppress inflammation via suppression of pro-inflammatory factors and induction of anti-inflammatory mediators, they can also independently drive bone loss by inhibiting differentiation and inducing apoptosis of osteoblasts whilst increasing osteoclast differentiation by stimulating expression of RANKL and decreasing its decoy receptor osteoprotegerin (OPG). Osteoblasts (OBs), p38 mitogen-activated protein kinases (p-38-MAPK), glucocorticoid-induced leucine zipper (GILZ), secretory leukocyte protease inhibitor (SLPI), tristetraprolin (TTP), mitogen-activated protein kinase-1 (MKP-1), activator protein 1 (AP-1), OC (osteoclast), canonical WNT signalling (WNT), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB).