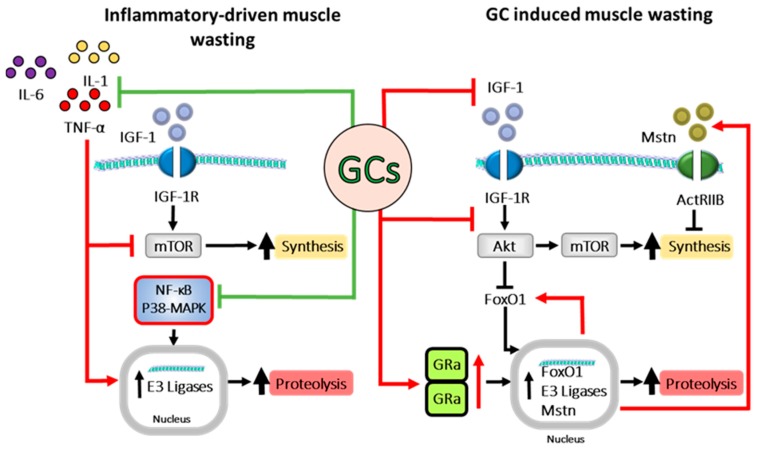
Figure 3.
Schematic representation of signalling pathways involved in both inflammatory driven and GC induced muscle wasting and their interactions. Inflammatory cytokines such as TNF-α and IL-1 inhibit mammalian target of rapamycin (mTOR) signalling, dampening muscle protein synthesis, whilst simultaneously inducing transcription of E3 ligases, leading to muscle proteolysis. Glucocorticoids (GCs) inhibit inflammatory signalling, including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signalling, and therefore decrease inflammatory driven muscle wasting. Despite this, GCs also drive muscle wasting through several pathways themselves, including suppression of the IGF-1/AKT/mTOR signalling cascade, leading to decreased protein synthesis and increased FOXO1 transcription. GR activation and dimerization induce the transcription of myostatin (MSTN), FOXO1 and other E3 ligases, leading to increased proteolysis and diminished protein synthesis. Forkhead box protein O1 (FOXO1), mammalian target of rapamycin (mTOR), insulin like growth factor (IGF-1), (p-38-MAPK), glucocorticoid induced leucine zipper (GILZ), secretory leukocyte protease inhibitor (SLPI), tristetraprolin (TTP), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), glucocorticoid receptor (GR), myostatin (MSTN), and IGF-1 receptor (IGF-1 R).