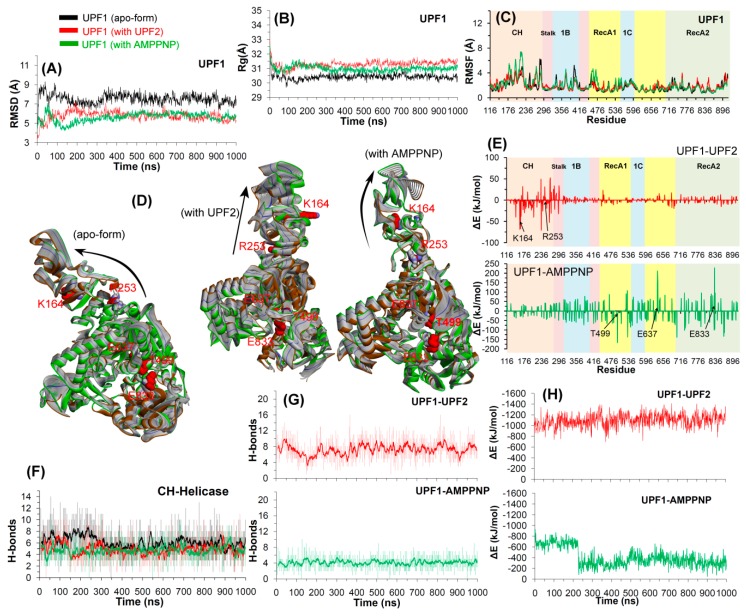
Figure 3.
Structural analysis of wild-type UPF1 in the MD simulations. (A–C) RMSD, radius of gyration profile, and RMSF representing the structural changes and fluctuations of residues inthe UPF1 protein. (D) The protein motion corresponding to the first eigenvector defined on the basis of the combined trajectories (green is from the beginning, blue from 400 ns (where the system stabilized), and brown from the end of the MD). (E,H) Energy contribution of each UPF1 residue to the binding with UPF2/AMPPNP and the total binding energy for UPF1-UPF2/AMPPNP, calculated using MM-PBSA. Contribution of residues selected for mutation analysis are labelled and shown with black arrows in (E). (F,G) Number of hydrogen bonds formed between the CH-helicase domains (intramolecular UPF1) and between UPF1-UPF2/AMPPNP (intermolecular). The dark lines represent the moving average of H-bonds formed with a period of 10 ns (i.e., number of H-bonds averaged every 10 ns).