Fig. 4.
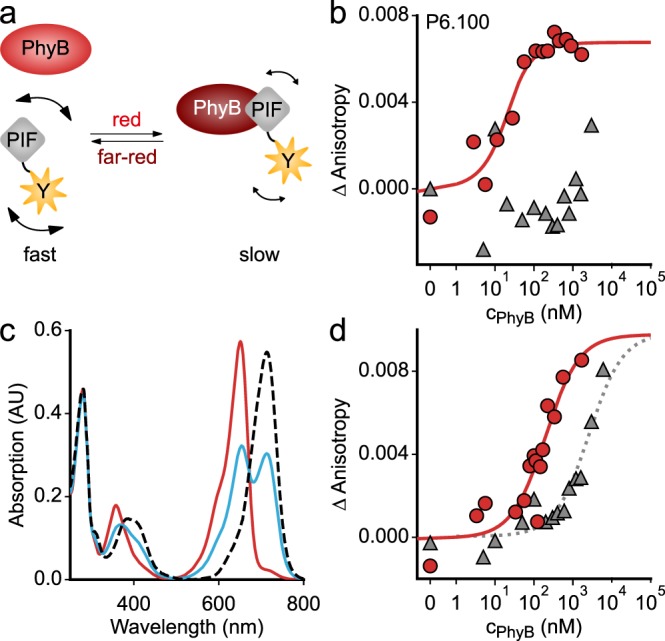
Quantitative analyses of the light-dependent protein:protein interaction between AtPIF variants and the AtPhyB PCM. a In its Pr state, the AtPhyB PCM exhibits weak or no affinity to AtPIF, but upon red-light exposure, the affinity is enhanced. Binding to the AtPhyB PCM increases the effective hydrodynamic radius of the AtPIF variants and slows down rotational diffusion. In turn, the fluorescence anisotropy of an EYFP tag C-terminally appended to the AtPIF increases. b Titration of 20 nM P6.100-EYFP with increasing concentrations of dark-adapted (gray) or red-light-exposed AtPhyB PCM (red), as monitored by anisotropy of the EYFP fluorescence. Data points show mean of n = 3 biological replicates. The red line denotes a fit to a single-site-binding isotherm. c Absorption spectra of the AtPhyB PCM in its dark-adapted Pr state (red line) and as a Pfr/Pr mixture following red-light exposure (blue). The dashed line denotes the absorption spectrum of the pure Pfr state, calculated according to ref. 42. d As in b but for P3.100-EYFP rather than P6.100-EYFP. Experiments were repeated twice with similar results.
