Abstract
Recently, the CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) showed the successful anti-inflammatory benefit of canakinumab, a monoclonal antibody targeting interleukin-1ß (IL-1ß) towards major cardiovascular events (MACE) in patients with a previous myocardial infarction. The magnitude of reduction in MACE was directly attributed to a reduction witnessed in IL-6 and CRP and highlighted the therapeutic potential of selectively targeting IL-1ß for atherosclerotic disease, a notion previously introduced in animal models. IL-1ß is involved in the downstream activation of the IL-6 receptor, which itself has been previously implicated as a target for atherothrombosis from Mendelian randomization studies. Further support has been garnered with the results of CIRT (Cardiovascular Inflammation Reduction Trial), which showed the inability of low-dose methotrexate to reduce IL-1ß, IL-6, or hsCRP in addition to MACE among patients with prior MI or multivessel coronary artery disease (CAD) but with normal hsCRP levels. Therefore, elucidation of therapeutic targets against the IL-1ß pathway are of immense interest currently in treating atherothrombosis. Upstream and serving as an activator of IL-1ß lies the nucleotide-binding oligomerization domain-like (NOD) receptor protein 3 (NLRP3) inflammasome that has been well described in animal models to be activated by cholesterol crystals or hypoxia to promote cleavage and secretion of IL-1ß and IL-18 that lead to atherosclerotic deposition in arteries. Given the direct implication of an atherogenic role to the NLRP3 inflammasome in generating these cytokines, NLRP3 inhibitors are of interest with the consideration to move upstream from the initial success of anti-IL-1ß therapy. With further discussion of the existing knowledge on the proinflammatory relationship of the NLRP3 inflammasome with atherosclerosis, this review summarizes and critically evaluates the preclinical and interventional findings of endogenous NLRP3 inflammasome inhibition in attempts to elucidate anti-inflammatory mechanisms and therapeutic targets against atherothrombosis. Further investigation focusing on the endogenous mechanisms of inhibition of the NLRP3 inflammasome would uncover diagnostic routes from defective means in inflammatory resolution. Specifically, pro-resolving lipid mediators, autophagy, and phosphorylation/dephosphorylation mechanisms are three points of worthy investigation from existing evidence.
Keywords: Atherosclerosis, Inflammasomes, Inflammation, NLRP3, Specialized Pro-resolving mediators, Thrombosis
1. Introduction:
From merely a matter of lipid dysregulation, evidence of residual inflammatory risk towards atherosclerosis has shifted the paradigm in understanding its pathophysiology and therapeutic options.1–2 Results from two randomized clinical trials, PROVE-IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy – Thrombolysis in Myocardial Infarction 22) and JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin), have highlighted residual inflammatory risk with evidence of reduced rates of major adverse cardiovascular events (MACE) via reductions in high sensitivity C-reactive protein (hsCRP) and independent of reductions in low density lipoprotein cholesterol (LDL-C).3–4 Recently, the CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) showed the successful anti-inflammatory benefit of canakinumab (150mg every 3 months, subcutaneously), a monoclonal antibody targeting interleukin-1ß (IL-1ß) that is approved for rheumatologic diseases, towards MACE among patients with a previous myocardial infarction (MI).5 The magnitude of reduction in MACE was directly attributed to a reduction witnessed in IL-6 (32% reduction with post-treatment IL-6 level below 1.65 ng/L) and hsCRP (25% reduction with post-treatment hsCRP concentrations less than 2 mg/L) and highlighted the therapeutic potential of selectively targeting IL-1ß for atherosclerotic disease, a notion previously introduced in animal models.6–10 IL-1ß is involved in the downstream activation of the IL-6 receptor, which itself has been previously implicated as a target for atherothrombosis from Mendelian randomization studies.11–12 Emphasis on inquiry towards this pathway relative to other inflammatory pathways has recently garnered further support with the results of CIRT(Cardiovascular Inflammation Reduction Trial), which showed the inability of low-dose methotrexate to reduce IL-1ß, IL-6, or hsCRP in addition to MACE among patients with prior MI or multivessel coronary artery disease (CAD) but with normal hsCRP levels.13 To this aim, in addition to resolving whether proinflammatory signatures of IL-1ß pathway selectively or ubiquitously predispose to atherosclerotic disease, elucidation of therapeutic targets within it are of immense interest currently.
Upstream and serving as an activator of IL-1ß lies the nucleotide-binding oligomerization domain-like (NOD) receptor protein 3 (NLRP3) inflammasome.14 With atherosclerotic deposition in arteries, the NLRP3 inflammasome has been well described in animal models to be activated by caspase-1 in the presence of cholesterol crystals or hypoxia to promote cleavage and secretion of IL-1ß and IL-18.15–16 Additionally, evidence of cholesterol crystal-induced activation of the NLRP3 inflammasome within macrophages and with hemodynamic-related activation of sterol regulatory element binding protein 2(SREBP2) provide evidence for a link between cholesterol metabolism and inflammation related to atherosclerosis.17–18 Given the direct implication of an atherogenic role to the NLRP3 inflammasome, NLRP3 inhibitors are of interest with the consideration to move upstream from the initial success of anti-IL-1ß therapy. With further discussion of the existing knowledge on the proinflammatory relationship of the NLRP3 inflammasome with atherosclerosis, this review summarizes and critically evaluates the preclinical and interventional findings of endogenous NLRP3 inflammasome inhibition in attempts to elucidate anti-inflammatory mechanisms and targets against atherothrombosis.
2. NLRP3 Inflammasome Pathway and Atherosclerosis:
The NLRP3 inflammasome is composed of the NLRP3 protein (also known as cryopyrin and NLRP3), one of five germline-encoded pattern-recognition receptors (PRRs) known to assemble a canonical inflammasome, which is distinguished in its ability to convert pro-caspase-1 into the active caspase-1.19–20 In addition to these two components of the NLRP3 inflammasome, the adaptor protein ASC is a third necessary component, and is required for the interaction with caspase-1 via its caspase recruitment domain (CARD).21 Interestingly, NEK7, a serine/threonine kinase involved in mitosis, has recently been implicated non-simultaneously as an additional component of NLRP3 inflammasome activation by binding to its leucine-rich repeat domain to promote assembly.22 Specific upstream mechanisms of activation of NLRP3 inflammasomes that have been described include potassium efflux, generation of reactive oxygen species (ROS), and lysosomal destabilization.23 Recently, post-translational modifications, including ubiquitination and phosphorylation have also been implicated as having a role in the activation process.24 Within macrophages, a priming event is first required in the activation of the NLRP3 inflammasome where an inflammatory stimulus promotes the necessary nuclear factor-kappa beta (NF-kß) signaling.25 The activation of the NF-kß pathway also promotes the expression of pro-IL-1ß and pro-IL18, both of which serve as substrates to be activated by the recruited caspase-1 activity of the NLRP3 inflammasome.26 A second activation step within macrophages either occurs sequentially or simultaneously to the initial priming step and involves the final assembly of the inflammasome to promote the catalytic action of its caspase component towards these inactive substrates.27 The latter activation step is mediated by the presence of pattern-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) to promote pyroptosis, a form of cell death that is mediated by the cleavage of gasdermin D (GSDMD) to increase plasma membrane permeability (by membrane pore formation).27–28 Pyroptosis is the juncture of canonical inflammasome activity (caspase-1 activation) and non-canonical inflammasome-induced activity through caspase-4, caspase-5 (humans) and caspase-11 (mice).27 Upon activation, IL-1ß promotes vascular smooth muscle (vSMC) proliferation in addition to increased leukocyte recruitment and adhesion, as well the upregulation of several proinflammatory cytokines, including fibrinogen, plasminogen activator inhibitor (PAI), IL-6, and hsCRP.29–31 The role of IL-18 with atherothrombosis was shown to upregulate interferon gamma in macrophages and NK cells for plaque formation and likewise increase collagenolytic activity of vSMC to promote plaque instability.32–33 Therefore, this pathway between the NLRP3 inflammasome to IL-1ß and then moving downstream to IL-6 and hsCRP is the center of an ongoing discussion with atherothrombosis.
Atherosclerosis involves a chronic inflammatory state that involves proliferation of vSMC, infiltration of leukocytes, endothelial dysfunction, and perpetuated lipid accumulation.34–35 The proinflammatory or proatherogenic mediators involved include IL-1ß, IL-6, IL-18, and tumor necrosis factor (TNF) that overwhelm anti-inflammatory mediators that include IL-10, IL-19, IL-1 receptor antagonist (IL-1RA) and IL-33.36–37 The earliest evidence of the NLRP3 inflammasome with atherosclerosis was shown by Duewell et al., where cholesterol crystals secondary to a diet-induced atherogenic diets in mice induced inflammation via caspase-1 activation by upregulated NLRP3 inflammasome activity in macrophages.38 It should be noted that such cholesterol crystal formation can also occur with the uptake of oxidized LDL by a scavenger receptor CD36 on phagolysosomes with intracellular crystallization.39 With the phagocytosis of cholesterol crystals by macrophages, Duewell et al. found that this led to phagolysosomal destabilization and rupture as phagocytosis could not continue. From this lysosomal rupture, leakage of the lysosomal enzyme cathepsin B was witnessed to further activate the NLRP3 inflammasome in human macrophages and has been discussed as an additional possible method of activation specific to atherosclerosis.40–41 Interestingly, Duewell et al. also found that mice transplanted with deficient NLRP3 were shown to have markedly decreased early atherosclerosis and inflammasome-dependent IL-18 levels. Further mice models of NLRP3 deficiency towards decreased atherosclerotic lesion size have emphasized the specific importance of a deficient caspase-1 gene expression, citing the means of reducing IL-1ß.42–44 Additionally, on the basis of intracellular crystallization and phagolysosomal destabilization, excess saturated fatty acids (palmitic acid and stearic acid) have been shown to similarly activate the NLRP3 inflammasome in murine primary macrophages in vitro and in mice in vivo.45 It is hypothesized by Karasawa et al. that since CD36 also serves as a fatty acid transporter, it may play a similar role in the incorporation of fatty acids within macrophages similar to what was evidenced with LDL cholesterol.45
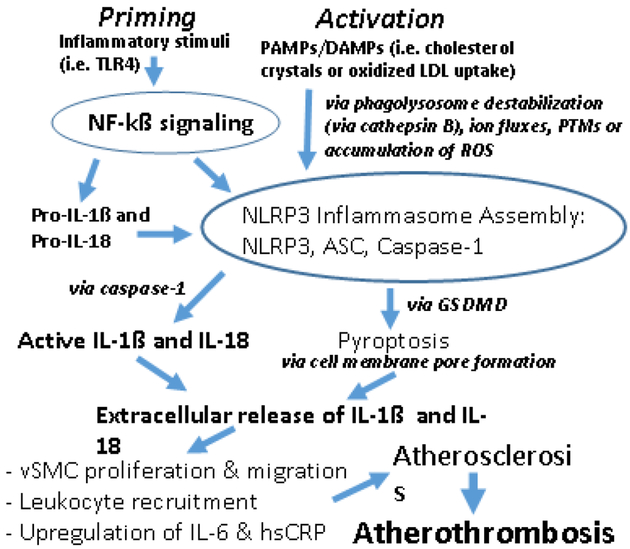
Considering the entirety of the NLRP3 pathway, epidemiologic human evidence from Mendelian randomization studies have identified IL-6 signaling to contribute to atherothrombosis.11,12 However, Mendelian Randomization studies of hsCRP have not been implicated similarly, and with no such data for IL-1ß, it has been said that a prime target for anti-atherothrombotic therapy is upstream to IL-6 (e.g. NLRP3 inflammasome).46 As described, CANTOS demonstrated the success of anti-IL-1ß therapy with canakinumab in the prevention of recurrent MACE in post-MI patients with residual inflammatory risk (hsCRP > 2mg/L).5 Certainly, the direct targeting of IL-6 is of interest with atheroprotection from the Mendelian Randomization studies and has engaged prospective evaluation. However, as noted from preliminary data from CANTOS, plasma IL-18 remained a determinant of residual risk despite this targeted anti-IL-1ß therapy and indicate a need to explore upstream with atherothrombosis.46 Therefore, in order to target both IL-1ß and IL-18, the upstream NLRP3 inflammasome is an attractive target and is mechanistically demonstrated in Figure 1. As an ongoing effort from these findings, below we discuss specific endogenous evidence with NLRP3 inhibition worth evaluation in the scope of atherothrombosis diagnostically and therapeutically.
Figure 1: NLRP3 Inflammasome Pathway and Atherosclerosis → Atherothrombosis.
Priming phase via NF-kß requires inflammatory stimuli (i.e. Toll-like receptor-4 [TLR-4]) to promote inflammasome assembly and subsequent active IL-1ß and IL-18. Activation phase is initiated via PAMPs/DAMPs (of note, cholesterol crystals or oxidized LDL uptake) and promotes NLRP3 inflammasome assembly and activation. Several NLRP3 inflammasome activation mechanisms have been noted, and phagolysosome destabilization (via cathepsin B) and post-translational modifications (PTMs) have been particularly described with atherosclerosis and atherothrombosis. NLRP3 inflammasome assembly also promotes pyroptosis via GSDMD which allows for the extracellular release of IL-1ß and IL-18 to initiate the recruitment of necessary inflammatory milieu and cytokines underlying atherosclerosis leading to atherothrombosis.
3. Endogenous Mechanisms of NLRP3 Inhibition and Atherosclerosis:
Pro-Resolving Lipid Mediators
Specialized pro-resolving mediators (SPMs) are a large superfamily of unsaturated fatty-acid derived lipid mediators that have been implicated and discussed in patients with atherosclerotic vascular disease.47–50 SPMs include lipoxins (LX), resolvins (Rv), protectins (P), and maresin (Mar) and serve as activators of G-protein coupled receptors.47,51–53 Within animal models, atherosclerosis has been shown to be a failure in inflammation resolution secondary to a deficiency in the 12/15-lipooxygenase (LOX) synthetic pathways of these SPMs.53 Interestingly, the localization of 5-LOX is critical to the utilization of arachidonic acid (AA) substrate by 12/15-LOX.54–55 Nuclear localization of 5-LOX is associated with the formation of proinflammatory leukotriene B4 (LTB4) by its own catalytic action and can potentiate such a necessary state in an atherogenic process.52 However, non-nuclear localization of 5-LOX near 12/15-LOX can help facilitate the conversion of AA to anti-atherogenic and anti-inflammatory SPMs.55 This conversion is believed to be facilitated by an efferocytosis receptor, MerTK.55–56 Likewise, the ratio of resolvin D1 (RvD1) to LTB4 was shown to be significantly reduced in human carotid plaques, a finding that was also appreciated with resolution deficits in RvD2 and Mar1 with vulnerable plaque rupture in ApoE −/− mice.57–58 The mechanisms of anti-atherogenic and anti-inflammatory resolution by SPMs have been best understood in animal models where stimulation of the A type LX (ALX)/formyl peptide receptor (FPR2) [ALX/FPR2] on vSMCs promotes attenuation of platelet-derived growth factor (PDGF)-stimulated vSMC migration and proliferation, increased M2 macrophage polarization, and decreased leukocyte recruitment.47,59–61 Interestingly, in animal models, exogenous delivery of SPMs was shown to counter the resolution deficit underpinning proinflammatory vascular injury.61–62
Despite direct evidence implicating the therapeutic potential of SPMs with atherosclerosis, this evidence has not been directly linked to modulation of the NLRP3 inflammasome. However, recent evidence of SPM involvement with attenuating the NLRP3 inflammasome outside of atherosclerosis has spurred interest in this direction in juncture with the findings of CANTOS. In bone marrow-derived macrophages from a mouse model challenged with lipopolysaccharide with adenosine triphosphate (ATP) or palmitate (activation mechanisms of NLRP3), RvD2 suppressed the expression of pro-IL-1ß and reduced the secretion of mature IL-1ß.63 The findings by Lopategi et al. were extended to their investigation in both thioglycolate-elicited peritoneal macrophages, where RvD2 hindered inflammasome priming events and subsequent activation events, including oligomerization of ASC, assembly, and caspase-1 activity, and in the in vivo inhibition of the NLRP3 inflammasome by RvD2 in a peritonitis model. Interestingly, IL-18 expression was also shown to be downregulated by RvD1 and RvD2, a necessary target as mentioned from the CANTOS findings. Interestingly and similar to the discussion noted with atherosclerosis, RvD2 effects were shown to be receptor mediated by Lopategi et al. as well. Further mechanistic understanding has been evidenced in a sepsis mouse model, where genetic NLRP3 deficiency conferred resolution of inflammation associated with polymicrobial sepsis by relieving caspase-dependent inhibition on lipoxin B4 (LXB4) synthesis in macrophages.64 In relation to anti-inflammatory efforts promoting inhibition of the NLRP3 inflammasome, mouse models of glomerular injury have found SPMs (RvD1 and Mar1) to suppress lipid raft redox signaling platforms and ROS generation.65–66 While the successful demonstration of exogenous SPM mediated inhibition of the NLRP3 inflammasome may offer a therapeutic approach stemming from these studies, SPMs do offer routes of intervention at the endogenous synthetic mechanisms described. In addition to this worthy inquiry, SPM deficiency may offer a diagnostic approach to identify potential responders to NLRP3 inhibition via exogenous SPM delivery towards atherothrombosis.
Autophagy
Autophagy is a highly conserved mechanism of intracellular degradation of toxic cytoplasmic material to the lysosomes for degradation.67 The delivery of these cytoplasmic substrates has been described in three ways: 1. Microautophagy, which utilizes an invagination of the lysosomal membrane, 2. Chaperone-mediated autophagy (CMA), which is dependent on a lysosomal-associated membrane protein 2 (LAMP2) to coordinate with other proteins to form a multi-protein channel necessary for substrate delivery to lysosomes, and 3. Macroautophagy, which is the best understood and assumed mechanism in the discussion of autophagy, involves double-membraned autophagosomes with substrate delivery via membrane fusion.67–69 With relation to the NLRP3 inflammasome, autophagy has been shown to be a negative regulator of its activation.70–71 Of the mechanisms described, clearance of damaged mitochondria (known as the mitophagy variant of autophagy) prevents the accumulation of mitochondrial ROS to activate the NLRP3 inflammasome.70 Specifically, both ROS generation and NLRP3 inflammasome activation was suppressed with inhibition of voltage-dependent anion channel related to mitochondrial activity. Furthermore, in a mouse macrophage model with M. tuberculosis infection, degradations of the assembled NLRP3 inflammasome complex was also shown to be mediated by ubiquitination occurring during autophagy, limiting early IL-1ß production.71
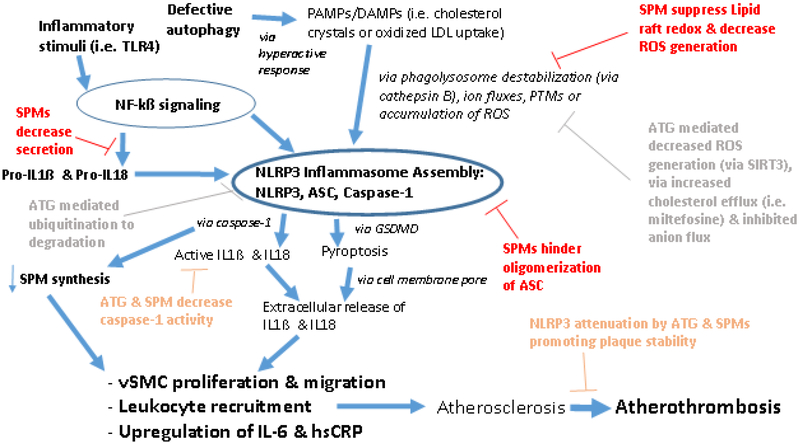
In relation to atherothrombosis, direct evidence exists that links plaque progression with NLRP3 inflammasome activity.71–75 In addition to finding evidence of autophagy markers colocalized with macrophages in atherosclerotic plaques, Razani et al. demonstrated the direct influence of autophagy influencing accelerated atherogenesis in an autophagy related 5 (ATG5) double knockout mouse model via enhanced NLRP3 inflammasome activation.71 Macrophage-specific ATG5-null mice are commonly used models for autophagy haploinsufficiency and deficiency.76 Razani et al. also note that the defective autophagy promotes an exaggerated response to cholesterol-crystal mediated hyperactivation of the NLRP3 inflammasome. Interestingly, they did not find that heterozygosity of autophagy deficiency was atherogenic, implicating the need to understand what degree of impairment is necessary. Similar findings of pro-atherogenic NLRP3 inflammasome activity were also demonstrated with a double knockout CD38 mouse model, a gene responsible for the regulation of lysosome function and autophagic flux.77 Mechanistically, the current direct evidence between defective autophagy and NLRP3 inflammasome mediated atherosclerosis has implicated sirtuin 3 (SIRT3), a nicotinamide adenine dinucleotide (NAD)-dependent deacetylase sensitive to metabolic status.78 Previously, the activation of NLRP3 inflammasome in ApoE −/− mice towards pro-atherogenic vascular inflammation was shown to be mediated by the inhibition of the SIRT3 pathway involving superoxide dismutase 2 (SOD2) and mitochondrial ROS signaling.79 In addition to finding that SIRT3 levels were reduced in peripheral monocytes from obese humans and palmitate-treated THP-1 (human monocytic cell line), corresponding to enhanced NLRP3 inflammasome and reduced autophagy, Liu et al. found that SIRT3 could form a molecular complex with ATG5. In such a reaction, SIRT3 overexpression altered the acetylation of endogenous ATG5, thereby inhibiting ROS generation and inhibiting the activation of the NLRP3 inflammasome. Given the direct evidence discussed here with autophagy defects leading to increased NLRP3 activity, focused exogenous interventions towards atherosclerosis such as miltefosine, an FDA approved drug for treating leishmaniasis, have been explored.80 In murine macrophages, miltefosine increased cholesterol efflux and induced autophagy in macrophages, promoting the dampening of the NLRP3 inflammasome and promoting lipid droplet degradation. As noted by Iacano et al., failure towards engulfment of modified lipids results in cholesterol crystal deposition underlying atherosclerotic plaque formation. Similar to our discussion regarding the successful demonstration of exogenous SPM mediated inhibition of the NLRP3 inflammasome, such findings of repair defects in autophagy may offer a therapeutic approach stemming from these studies. However, as reviewed by Galluzzi et al., modulators of autophagy are a complicated endeavor and require careful evaluation, including non-mutually exclusive mechanisms of disease, and the benefit of activation vs. recovery mechanisms of autophagy.81 Nevertheless, these defects in autophagy do also offer a diagnostic approach similar to SPM deficiency to identify potential responders to NLRP3 inhibition towards atherothrombosis (Fig. 2).
Figure 2: SPM (red, orange) and Autophagy [ATG] (gray, orange) Inhibitory Mechanisms of the NLRP3 Inflammasome Pathway and Potential Routes against Atherosclerosis → Atherothrombosis.
SPMs inhibitory mechanisms have been evidenced with decrease secretion of pro-IL-1ß and pro-IL-18, and likewise decreased IL-1ß and IL-18 (via diminished caspase activity). SPMs have also been shown to decrease NLRP3 inflammasome activation via suppressed lipid raft redox and decreased ROS generation, in addition to preventing the assembly of the inflammasome via hindering oligomerization of ASC. On a proinflammatory note, caspase-1 can diminish SPM synthesis. ATG inhibitory mechanisms against NLRP3 inflammasome activation have been evidenced with decreased ROS generation (noted via SIRT3), increased cholesterol efflux, and inhibited anion flux. Additionally, an assembled NLRP3 inflammasome complex can be ubiquitinated and degraded via autophagic mechanisms, and thereby decrease caspase 1 activity to diminish IL-1ß and IL-18 release. On a proinflammatory note, defective autophagy promotes a hyperactive response to PAMP/DAMPs and has been noted with cholesterol crystal substrate. Together, it is quite possible both SPMs and ATG offer routes to address atherosclerosis and atherothrombosis via these mechanisms described.
Post-translational modifications – Inhibitory Regulators
Among post-translational modifications, phosphorylation/dephosphorylation mechanisms related to the activation of the NLRP3 inflammasome have been well described with differing roles. Phosphorylation via several kinase systems, including TGF-ß-activated kinase1 (TAK1), mitogen-activated protein kinase kinase kinase (MAPKKK), c-Jun N-terminal kinase1 (JNK1), external signal regulated kinase1 (ERK1), PI-3K/Rac1/p21-activated kinase (PAK1) or other TLR signaling-related kinases have all been implicated as critical components in the modulation of NLRP3 priming or subsequent activation steps.82–85 Likewise, exogenous inhibitors to these kinases is a worthy endeavor therapeutically and may explain success noted with small molecule inhibitors such as MCC950.86 However such therapeutic mechanisms are out of the scope of this review. Focusing on endogenous negative regulators of NLRP3 (Table 1), inhibitor of kappa beta (IKK)α can phosphorylate both Ser16 and Ser193 of the ASC component of the NLRP3 inflammasome to induce its sequestration into the nucleus.87 This was found in contrast to the role of IKKi/IKKε which phosphorylate Ser58 reside of ASC to promote the translocation of ASC towards priming of the NLRP3 inflammasome. Interestingly, as Song et al. have indicated, the specific activation mechanisms of ASC and its subsequent perinuclear translocation need further investigation.
Table 1:
Intracellular targets in the post-translational modifications associated with endogenous negative inhibition of NLRP3 inflammasome priming or activation phases.
| Post-translational Modification Target | Phase of NLRP3 Inflammasome affected | References |
|---|---|---|
| (IKK)α | Priming via phosphorylation | 87 |
| PKA | Activation via phosphorylation | 91–93 |
| PTPN22 | Activation via dephosphorylation | 96 |
| TRIM31 | Activation via ubiquitination | 88 |
| ARIH2 | Activation via ubiquitination | 89 |
| MARCH17 | Activation via ubiquitination | 95 |
Moving to the activation and assembly phase of the NLRP3 inflammasome, which includes the oligomerization of ASC and cleavage of GSDMD, phosphatase of protein phosphatase 2A (PP2A) is responsible for the dephosphorylation of Ser5 on the N-terminal pyrin domain (PYD) of NLRP3, promoting recruitment of ASC.87 However, the endogenous kinase that phosphorylates Ser5 of PYD is not known and is worth investigation. Nevertheless, endogenous ubiquitination/deubiquitination mechanisms have been described to attenuate NLRP3 inflammasome activation. Within a colitis model, TRIM31, an E3 ubiquitin ligase, was shown to be a feedback suppressor of the NLRP3 inflammasome with stimulation of the protein theorized to be by the same IL-1ß product of inflammation.88 Similar negative regulation via ubiquitination of NLRP3 has been described with the E3 ubiquitin ligase Ariadne Homolog 2 (ARIH2) in murine peritoneal macrophages.89 Conversely, deubiquitination has been shown to be a positive regulator with the activation of the NLRP3 inflammasome via the cytosolic enzyme BRCC3-containing BRISC complex.90
Further known endogenous inhibitory mechanisms of post-translational modifications during the activation phase include protein kinase A (PKA) which was found to directly attenuate NLRP3 activation via phosphorylation of Ser295 of human NLRP3, which was mediated by prostaglandin E2 (PGE2) signaling via the PGE2 receptor E-prostanoid 4 (EP4).91 Likewise, as a part of the PKA signaling pathway, cyclic adenosine monophosphate (cAMP) can also directly inhibit the NLRP3 inflammasome at Ser295 of its receptor.92 Interestingly, mutations in Ser295 has been linked to the disease CAPS (cyropyrin-associated periodic syndromes).93 Despite lacking direct evidence of these mechanisms with atherosclerosis, activation of PKA via transmembrane G-protein-coupled receptor (TGR5), a bile acid receptor promoted phosphorylation of Ser291 of NLRP3, which led to its degradation via ubiquitination.92 To note, TGR5 agonists have been shown to inhibit oxidized LDL uptake in macrophages and halt atherosclerosis in a mouse model with TGR5 receptor response.94 Interestingly, within peritoneal inflammation, ubiquitination and degradation of NLRP3 via cAMP was shown to be mediated via the E3 ubiquitin ligase MARCH17, similar to the findings noted with TRIM31.95 Finally, protein tyrosine phosphatase, non-receptor type 22 (PTPN22) has been evidenced as another direct endogenous inhibitory mechanism against the activation phase that targets Tyr861 of NLRP3.96 However, the activity of PTPN22 has been localized to the lamina propria in the setting of inflammatory colitis due to tightly regulated mRNA expression, and thus its evidence in other cell types is currently unknown.97 Taken together, endogenous means of phosphorylation/dephosphorylation mechanisms specific to NLRP3 inflammasome regulation and atherothrombosis require further investigation as it may also offer additional diagnostic and therapeutic approaches to identify and help potential responders to NLRP3 inhibition.
4. Diagnostic and Therapeutic Implications:
The NLRP3 inflammasome is an attractive target towards future therapeutic investigation surrounding atherothrombosis. Certainly, endogenous proinflammatory mechanisms of the NLRP3 inflammasome are of interest. For example, some interest is currently with colchicine intervention, stemming from two large phase 3 clinical trials in the secondary prevention of cardiovascular disease.98–100 Likewise, the myriad of proposed activation mechanisms of the NLRP3 inflammasome require further mechanistic understanding and may offer additional points of targeted inhibition.101 However, focusing on the endogenous mechanisms of inhibition of the NLRP3 inflammasome, it may also be that such investigation uncovers diagnostic routes from defective means in inflammatory resolution. Among our discussion here, SPMs are one such point of interest. Separately, evidence has implicated their involvement in the resolution associated with atherosclerosis and the NLRP3 inflammasome, with an SPM deficiency offering a route diagnostically towards atherothrombosis. However, direct evidence of SPM modulation of NLRP3 inflammasome activity with atherosclerosis is needed and a worthy investigation. Additionally, by any intervention towards the inflammatory risk with atherothrombosis, anti-IL-18 therapy requires equal attention to anti-IL-1ß therapy as supported by the findings from CANTOS. Interestingly, SPMs do offer the possibility of attenuating both cytokines as discussed. Autophagy is a second means of endogenous inhibition of the NLRP3 inflammasome that we discussed. Tying intimately with the activation substrates (i.e. ROS and ion fluxes) autophagy provides links to the early regulatory steps surrounding activation of the NLRP3 inflammasome. Likewise, with direct but limited evidence towards atherosclerosis secondary to NLRP3 inflammasome activity, defective mechanisms of autophagy (reduced SIRT3) have offered a route to intervention (i.e. miltefosine) and diagnosis. However, mechanistic questions warrant further investigation prior to inquiry into targeted diagnosis or intervention via autophagy. For one, the degree of defective autophagy identified in animal models (double knockout) may not correspond or be viable to humans. Heterozygous evidence is limited. Therapeutically, autophagy stimulation is a worthy investigation, particularly by means of mitophagy, but will be need to evaluated for success relative to intervening with recovery mechanisms of autophagy. Finally, our discussion of post-translational modifications focused on well-described mechanisms of phosphorylation/dephosphorylation towards negative regulation of the NLRP3 inflammasome. Of these mechanisms, endogenous inhibition during the priming phase of NLRP3 inflammasome activation is less known but IKKα activity has proved the worth of such investigation. The modulation of ASC translocation by IKKα warrants further understanding of its proinflammatory movement during the priming phase. Similar to our discussion with SPMs, large evidence exists of phosphorylation/dephosphorylation mechanisms related to inhibitory action of the NLRP3 activation phase and separately, atherosclerosis (i.e. TGR5 agonists. Mechanistically, many of the phosphorylation/dephosphorylation evidence is from in vitro findings and support for those routes implicated require further in vivo evaluation. Defective mechanisms of necessary post-translational modifications offer an additional route diagnostically and stimulators of targeted kinase activity is a possibility towards inflammation surrounding atherothrombosis. Taken together, these three approaches may also highlight the need for a customized approached to NLRP3 inflammasome inhibition, should it be established as a viable therapy towards atherothrombosis.
5. Conclusion:
With the findings of CANTOS and CIRT, the NLRP3 inflammasome pathway with the modulation of IL-1ß and IL-18 is of further diagnostic and therapeutic interest as we branch from the residual inflammatory risk hypothesis associated with atherothrombosis. Endogenous mechanisms of NLRP3 inflammasome inhibition offer a large-scale opportunity to understand intervention as twofold, attenuating proinflammation routes or promoting pro-resolving mechanisms. Defective and deficient mechanisms of NLRP3 inflammation resolution provides diagnostic understanding as well. Additionally, further elucidation of the specific mechanisms of the NLRP3 inflammasome with atherothrombosis is necessary to refine what will be a broader therapeutic need compared to other established cryopyrin pathologies.
Acknowledgement
This work was supported by research grants R01 HL144125, R01 HL120658, and R01HL147662 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All authors have read the journal’s authorship agreement.
Abbreviations
- ALX
A type lipoxin receptor
- ASC
apoptosis-associated speck-like protein containing CARD
- ATG5
autophagy related 5
- CAPS
cryopyrin-associated periodic syndromes
- CARD
caspase activation and recruitment domain
- ERK1
external signal regulated kinase1
- FPR2
N-formyl peptide receptor-2
- GSDMD
gasdermin D
- IL-1ß
interleukin-1ß
- JNK1
c-Jun N-terminal kinase1
- LAMP2
lysosomal-associated membrane protein 2
- LRR
leucine-rich repeats
- MAPKKK
mitogen-activated protein kinase kinase kinase
- Mar
Maresin
- NEK7
NIMA-related kinase 7
- NIMA
never in mitosis gene a
- NLRP3
NOD-, LRR- and pyrin domain-containing protein-3
- NOD
nucleotide-binding oligomerization domain
- PAK1
PI-3K/Rac1/p21-activated kinase
- Rv
resolvin
- SPMs
specialized pro-resolving lipid mediators
- SIRT3
sirtuin 3
- TAK1
TGF-ß-activated kinase1
- TGR5
transmembrane G-protein-coupled receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests’ disclosure
Both authors have read the journal’s policy on disclosure of potential conflicts of interest. The authors have no other relevant affiliations or financial or non-financial involvement with any organization or entity with financial or non-financial interest or conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References:
- [1].Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. New England Journal of Medicine. 2005;352(16):1685–1695. doi: 10.1056/nejmra043430. [DOI] [PubMed] [Google Scholar]
- [2].Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- [3].Ridker PM, Cannon CP, Morrow D, et al. C-Reactive Protein Levels and Outcomes after Statin Therapy. New England Journal of Medicine. 2005;352(1):20–28. doi: 10.1056/nejmoa042378. [DOI] [PubMed] [Google Scholar]
- [4].Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. New England Journal of Medicine. 2008;359(21):2195–2207. doi: 10.1056/nejmoa0807646. [DOI] [PubMed] [Google Scholar]
- [5].Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New England Journal of Medicine. 2017;377(12):1119–1131. doi: 10.1056/nejmoa1707914. [DOI] [PubMed] [Google Scholar]
- [6].Kirii H, Niwa T, Yamada Y, et al. Lack of Interleukin-1β Decreases the Severity of Atherosclerosis in ApoE-Deficient Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(4):656–660. doi: 10.1161/01.atv.0000064374.15232.c3. [DOI] [PubMed] [Google Scholar]
- [7].Shimokawa H, Ito A, Fukumoto Y, et al. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest. 1996;97(3):769–776. doi: 10.1172/JCI118476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sager HB, Heidt T, Hulsmans M, et al. Targeting Interleukin-1β Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation. 2015;132(20):1880–1890. doi: 10.1161/CIRCULATIONAHA.115.016160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ridker PM, Libby P, Macfadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study(CANTOS). European Heart Journal. 2018;39(38):3499–3507. doi: 10.1093/eurheartj/ehy310. [DOI] [PubMed] [Google Scholar]
- [10].Ridker PM, Macfadyen JG, Everett BM, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. The Lancet. 2018;391(10118):319–328. doi: 10.1016/s0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- [11].Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium, Swerdlow DI, Holmes MV, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379(9822):1214–1224. doi: 10.1016/S0140-6736(12)60110-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].IL6R Genetics Consortium Emerging Risk Factors Collaboration, Sarwar N, Butterworth AS, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379(9822):1205–1213. doi: 10.1016/S0140-6736(11)61931-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-Dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. (2019) 380:752–62. doi: 10.1056/NEJMoa1809798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ridker PM. From CRP to IL-6 to IL-1: moving upstream to identify novel targets for atheroprotection. Circ Res. (2016) 118:145–56. doi: 10.1161/CIRCRESAHA.115.306656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals [published correction appears in Nature. 2010 Jul 29;466(7306):652]. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Folco EJ, Sukhova GK, Quillard T, Libby P. Moderate hypoxia potentiates interleukin-1β production in activated human macrophages. Circ Res. 2014;115(10):875–883. doi: 10.1161/CIRCRESAHA.115.304437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiao H, Lu M, Lin TY, et al. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128(6):632–642. doi: 10.1161/CIRCULATIONAHA.113.002714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rajamäki K, Lappalainen J, Oörni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5(7):e11765 Published 2010 Jul 23. doi: 10.1371/journal.pone.0011765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sharma D, Kanneganti TD. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol. 2016;213(6):617–629. doi: 10.1083/jcb.201602089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2015;16(1):7–21. doi: 10.1038/nri.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schroder K, Zhou R, Tschopp J. The NLRP3 Inflammasome: A Sensor for Metabolic Danger? Science. 2010;327(5963):296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- [22].Shi H, Wang Y, Li X, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nature Immunology. 2015;17(3):250–258. doi: 10.1038/ni.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. doi: 10.1038/nm.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang Z, Meszaros G, He WT, et al. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J Exp Med. 2017;214(9):2671–2693. doi: 10.1084/jem.20162040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–791. doi: 10.4049/jimmunol.0901363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kesavardhana S, Kanneganti TD. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int Immunol. 2017;29(5):201–210. doi: 10.1093/intimm/dxx018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- [28].Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- [29].Libby P Interleukin-1 Beta as a Target for Atherosclerosis Therapy. Journal of the American College of Cardiology. 2017;70(18):2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bevilacqua MP, Pober JS, Majeau GR, Fiers W, Cotran RS, Gimbrone MA. Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proceedings of the National Academy of Sciences. 1986;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Libby P, Warner SJ, Friedman GB. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988;81(2):487–498. doi: 10.1172/JCI113346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nooijer RD Thüsen J.h. Von Der, Verkleij C, et al. Overexpression of IL-18 Decreases Intimal Collagen Content and Promotes a Vulnerable Plaque Phenotype in Apolipoprotein-E–Deficient Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(12):2313–2319. doi: 10.1161/01.atv.0000147126.99529.0a. [DOI] [PubMed] [Google Scholar]
- [33].Tenger C, Sundborger A, Jawien J, Zhou X. IL-18 Accelerates Atherosclerosis Accompanied by Elevation of IFN-γ and CXCL16 E pression Independently of T Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(4):791–796. doi: 10.1161/01.atv.0000153516.02782.65. [DOI] [PubMed] [Google Scholar]
- [34].Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15(2):104–116. doi: 10.1038/nri3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118(4):692–702. doi: 10.1161/CIRCRESAHA.115.306361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415 [DOI] [PubMed] [Google Scholar]
- [37].Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals [published correction appears in Nature. 2010 Jul 29;466(7306):652]. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14(8):812–820. doi: 10.1038/ni.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rajamaki K, Lappalainen J, Oorni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One, 2010; 5: e11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Karasawa T, Takahashi M. Role of NLRP3 Inflammasomes in Atherosclerosis. Journal of Atherosclerosis and Thrombosis. 2017;24(5):443–451. doi: 10.5551/jat.rv17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Usui F, Shirasuna K, Kimura H, et al. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochemical and Biophysical Research Communications. 2012;425(2):162–168. doi: 10.1016/j.bbrc.2012.07.058. [DOI] [PubMed] [Google Scholar]
- [43].Gage J, Hasu M, Thabet M, Whitman SC. Caspase-1 Deficiency Decreases Atherosclerosis in Apolipoprotein E-Null Mice. Canadian Journal of Cardiology. 2012;28(2):222–229. doi: 10.1016/j.cjca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- [44].Hendrikx T, Jeurissen MLJ, Gorp PJV, et al. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development in Ldlr−/−mice. FEBS Journal. 2015;282(12):2327–2338. doi: 10.1111/febs.13279. [DOI] [PubMed] [Google Scholar]
- [45].Karasawa T, Kawashima A, Usui-Kawanishi F, et al. Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome inMacrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(4):744–756. doi: 10.1161/atvbaha.117.310581. [DOI] [PubMed] [Google Scholar]
- [46].Ridker PM. Anticytokine Agents. Circulation Research. 2019;124(3):437–450. doi: 10.1161/circresaha.118.313129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ho KJ, Spite M, Owens CD, et al. Aspirin-Triggered Lipoxin and Resolvin E1 Modulate Vascular Smooth Muscle Phenotype and Correlate with Peripheral Atherosclerosis. The American Journal of Pathology. 2010;177(4):2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. The FASEB Journal. 2016;30(8):2792–2801. doi: 10.1096/fj.201500155r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Satish M, Agrawal DK. Pro-resolving lipid mediators in the resolution of neointimal hyperplasia pathogenesis in atherosclerotic diseases. Expert Review of Cardiovascular Therapy. 2019;17(3):177–184. doi: 10.1080/14779072.2019.1563483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Buckley CD, Gilroy DW, Serhan CN. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity. 2014;40(3):315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Thorp EB. Proresolving Lipid Mediators Restore Balance to the Vulnerable Plaque. Circulation Research. 2016;119(9):972–974. doi: 10.1161/circresaha.116.309794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. The FASEB Journal. 2008;22(10):3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Luo M, Jones SM, Peters-Golden M, Brock TG. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proceedings of the National Academy of Sciences. 2003;100(21):12165–12170. doi: 10.1073/pnas.2133253100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fredman G, Ozcan L, Spolitu S, et al. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4synthesis by inhibiting a calcium-activated kinase pathway. Proceedings of the National Academy of Sciences. 2014;111(40):14530–14535. doi: 10.1073/pnas.1410851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cai B, Thorp EB, Doran AC, et al. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proceedings of the National Academy of Sciences. 2016;113(23):6526–6531. doi: 10.1073/pnas.1524292113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nature Communications. 2016;7(1). doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Viola JR, Lemnitzer P, Jansen Y, et al. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circulation Research. 2016;119(9):1030–1038. doi: 10.1161/circresaha.116.309492. [DOI] [PubMed] [Google Scholar]
- [59].Ho KJ, Spite M, Owens CD, et al. Aspirin-Triggered Lipoxin and Resolvin E1 Modulate Vascular Smooth Muscle Phenotype and Correlate with Peripheral Atherosclerosis. The American Journal of Pathology. 2010;177(4):2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. The FASEB Journal. 2015;29(6):2504–2513. doi: 10.1096/fj.14-265363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Liu G, Gong Y, Zhang R, et al. Resolvin E1 attenuates injury-induced vascular neointimal formation by inhibition of inflammatory responses and vascular smooth muscle cell migration. The FASEB Journal. 2018;32(10):5413–5425. doi: 10.1096/fj.201800173r. [DOI] [PubMed] [Google Scholar]
- [62].Wu B, Mottola G, Chatterjee A, et al. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. Journal of Vascular Surgery. 2017;65(1). doi: 10.1016/j.jvs.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lopategi A, Flores-Costa R, Rius B, et al. Frontline Science: Specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. Journal of Leukocyte Biology. 2018;105(1):25–36. doi: 10.1002/jlb.3hi0517-206rr. [DOI] [PubMed] [Google Scholar]
- [64].Lee S, Nakahira K, Dalli J, et al. NLRP3 Inflammasome Deficiency Protects against Microbial Sepsis via Increased Lipoxin B4 Synthesis. American Journal of Respiratory and Critical Care Medicine. 2017;196(6):713–726. doi: 10.1164/rccm.201604-0892oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Li G, Chen Z, Bhat OM, et al. NLRP3 inflammasome as a novel target for docosahexaenoic acid metabolites to abrogate glomerular injury. Journal of Lipid Research. 2017;58(6):1080–1090. doi: 10.1194/jlr.m072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tang S, Gao C, Long Y, et al. Maresin 1 Mitigates High Glucose-Induced Mouse Glomerular Mesangial Cell Injury by Inhibiting Inflammation and Fibrosis. Mediators of Inflammation. 2017;2017:1–11. doi: 10.1155/2017/2438247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Noda NN, Inagaki F. Mechanisms of autophagy. Annu Rev Biophys. 2015;44:101–122. doi: 10.1146/annurev-biophys-060414-034248 [DOI] [PubMed] [Google Scholar]
- [68].Li W-W, Li J, Bao J-K. Microautophagy: lesser-known self-eating. Cellular and Molecular Life Sciences. 2011;69(7):1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2013;24(1):92–104. doi: 10.1038/cr.2013.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2010;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- [71].Shi C-S, Shenderov K, Huang N-N, et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nature Immunology. 2012;13(3):255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Razani B, Feng C, Coleman T, et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15(4):534–544. doi: 10.1016/j.cmet.2012.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kockx MM, Meyer GRYD, Buyssens N, Knaapen MWM, Bult H, Herman AG. Cell Composition, Replication, and Apoptosis in Atherosclerotic Plaques After 6 Months of Cholesterol Withdrawal. Circulation Research. 1998;83(4):378–387. doi: 10.1161/01.res.83.4.378. [DOI] [PubMed] [Google Scholar]
- [74].Martinet W, Bie MD, Schrijvers DM, Meyer GRD, Herman AG, Kockx MM. 7-Ketocholesterol Induces Protein Ubiquitination, Myelin Figure Formation, and Light Chain 3 Processing in Vascular Smooth Muscle Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(12):2296–2301. doi: 10.1161/01.atv.0000146266.65820.a1. [DOI] [PubMed] [Google Scholar]
- [75].Martinet W, Meyer GRD. Autophagy in Atherosclerosis. Circulation Research. 2009;104(3):304–317. doi: 10.1161/circresaha.108.188318. [DOI] [PubMed] [Google Scholar]
- [76].Yoshii SR, Kuma A, Mizushima N. Transgenic rescue of Atg5-null mice from neonatal lethality with neuron-specific expression of ATG5: Systemic analysis of adult Atg5-deficient mice. Autophagy. 2017;13(4):763–764. doi: 10.1080/15548627.2017.1280221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yuan X, Bhat OM, Meng N, Lohner H, Li P-L. Protective Role of Autophagy in Nlrp3 Inflammasome Activation and Medial Thickening of Mouse Coronary Arteries. The American Journal of Pathology. 2018;188(12):2948–2959. doi: 10.1016/j.ajpath.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Liu P, Huang G, Wei T, et al. Sirtuin 3-induced macrophage autophagy in regulating NLRP3 inflammasome activation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2018;1864(3):764–777. doi: 10.1016/j.bbadis.2017.12.027. [DOI] [PubMed] [Google Scholar]
- [79].Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. Journal of the American Heart Association. 2017;6(11). doi: 10.1161/jaha.117.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Iacano AJ, Lewis H, Hazen JE, Andro H, Smith JD, Gulshan K. Miltefosine increases macrophage cholesterol efflux and inhibits NLRP3-inflammasome assembly and IL-1beta release. 2018. doi: 10.1101/230769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16(7):487–511. doi: 10.1038/nrd.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gong Y-N, Wang X, Wang J, et al. Chemical probing reveals insights into the signaling mechanism of inflammasome activation. Cell Research. 2010;20(12):1289–1305. doi: 10.1038/cr.2010.135. [DOI] [PubMed] [Google Scholar]
- [83].Song N, Liu Z-S, Xue W, et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Molecular Cell. 2017;68(1). doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- [84].Ghonime MG, Shamaa OR, Das S, et al. Inflammasome Priming by Lipopolysaccharide Is Dependent upon ERK Signaling and Proteasome Function. The Journal of Immunology. 2014;192(8):3881–3888. doi: 10.4049/jimmunol.1301974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Basak C, Pathak SK, Bhattacharyya A, Mandal D, Pathak S, Kundu M. NF-κB-and C/EBPβ-driven Interleukin-1β Gene E pression and PAK1-mediated Caspase-1 Activation Play Essential Roles in Interleukin-1β Release fromHelicobacter pyloriLipopolysaccharide-stimulated Macrophages. Journal of Biological Chemistry. 2004;280(6):4279–4288. doi: 10.1074/jbc.m412820200. [DOI] [PubMed] [Google Scholar]
- [86].Coll RC, Robertson AAB, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nature Medicine. 2015;21(3):248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Martin BN, Wang C, Willette-Brown J et al. IKKα negatively regulates ASC-dependent inflammasome activation. Nat Commun. 2014;5:4977 Published 2014 Sep 30. doi: 10.1038/ncomms5977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Song H, Liu B, Huai W, et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat Commun. 2016;7:13727 Published 2016 Dec 8. doi: 10.1038/ncomms13727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kawashima A, Karasawa T, Tago K, et al. ARIH2 Ubiquitinates NLRP3 and Negatively Regulates NLRP3 Inflammasome Activation in Macrophages. The Journal of Immunology. 2017;199(10):3614–3622. doi: 10.4049/jimmunol.1700184. [DOI] [PubMed] [Google Scholar]
- [90].Py BF, Kim M-S, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 Critically Regulates Inflammasome Activity. Molecular Cell. 2013;49(2):331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- [91].Mortimer L, Moreau F, Macdonald JA, Chadee K. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nature Immunology. 2016;17(10):1176–1186. doi: 10.1038/ni.3538. [DOI] [PubMed] [Google Scholar]
- [92].Guo C, Xie S, Chi Z, et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45(4):802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- [93].Lee GS, Subramanian N, Kim AI, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–127. doi: 10.1038/nature11588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Pols TW, Nomura M, Harach T, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14(6):747–757. doi: 10.1016/j.cmet.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yan Y, Jiang W, Liu L, et al. Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell. 2015;160(1–2):62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- [96].Bae JY, Park HH. Crystal Structure of NALP3 Protein Pyrin Domain (PYD) and Its Implications in Inflammasome Assembly. Journal of Biological Chemistry. 2011;286(45):39528–39536. doi: 10.1074/jbc.m111.278812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Spalinger MR, Kasper S, Gottier C, et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22 [published correction appears in J Clin Invest. 2016 Nov 1;126(11):4388]. J Clin Invest. 2016;126(5):1783–1800. doi: 10.1172/JCI83669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006. 440:237–41. doi: 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- [99].Martínez GJ, Robertson S, Barraclough J, et al. Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome. J Am Heart Assoc. 2015;4(8):e002128 Published 2015 Aug 24. doi: 10.1161/JAHA.115.002128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Martínez G Celermajer D Patel S. Corrigendum to: “The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation” [Atherosclerosis. 2018 Feb;269:262–271]. Atherosclerosis. 2018;273:157. doi: 10.1016/j.atherosclerosis.2018.03.043. [DOI] [PubMed] [Google Scholar]
- [101].Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death & Disease. 2019;10(2). doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]