Abstract
Purpose
To compare mycophenolate mofetil (MMF) to methotrexate (MTX) as corticosteroid-sparing therapy for ocular inflammatory diseases.
Design
Retrospective analysis of cohort study data.
Methods
Participants were identified from the Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study. Demographic and clinical characteristics were obtained via medical record review. The study included 352 patients who were taking single agent immunosuppression with MTX or MMF at four tertiary uveitis clinics. Marginal structural models (MSM)-derived statistical weighting created a virtual population with covariates and censoring patterns balanced across alternative treatments. With this methodological approach, the results estimate what would have happened had none of the patients stopped their treatment. Survival analysis with stabilized MSM-derived weights simulated a clinical trial comparing MMF vs. MTX for non-infectious inflammatory eye disorders. The primary outcome was complete control of inflammation on prednisone ≤10 mg/day, sustained for ≥30 days.
Results
The time-to-success was shorter (more favorable) for MMF than MTX (HR=0.68, 95% confidence interval: 0.46–0.99). Adjusting for covariates, the proportion achieving success was higher at every point in time for MMF than MTX from 2–8m then converges at 9 months. The onset of corticosteroid-sparing success took more than three months for most patients in both groups. Outcomes of treatment (MMF vs. MTX) were similar across all anatomic sites of inflammation. The incidence of stopping therapy for toxicity was similar in both groups.
Conclusions
Our results suggest that, on average, MMF may be faster than MTX in achieving corticosteroid-sparing success in ocular inflammatory diseases.
Corticosteroids have been the mainstay in the treatment of non-infectious ocular inflammatory diseases. The addition of immunosuppressive agents is indicated as an alternative to corticosteroids, and to reduce the incidence of corticosteroid-induced systemic side effects.1 In addition some ocular inflammatory diseases typically have an insufficient response to corticosteroids alone and fare better with use of immunosuppressive agents.1 A prospective multicenter randomized controlled trial comparing systemic anti-inflammatory therapy versus intraocular fluocinolone acetonide implant for active or recently active intermediate, posterior, and panuveitis, found minimal difference in systemic adverse effects in either group at 2-year, 4.5-year and 7-year follow up,2–4 with better visual outcomes by 7 years follow-up in the systemic group, suggesting systemic therapy should be the first line of treatment in most intermediate, posterior and panuveitis cases. In the systemic group, 104/126, 86% of the subjects were using corticosteroid sparing agents4,5 In this study, most patients receiving immunosuppression received methotrexate or mycophenolate mofetil. Retrospective data from a cohort summarizing over 60,000 person-years showed that the risk of mortality or cancer related mortality for persons treated with methotrexate or mycophenolate was similar to that of persons who never had been exposed to immunosuppressive agents. These studies show that systemic immunosuppression is effective and well tolerated and can be administered safely to control ocular inflammation.7.
Given that methotrexate and mycophenolate mofetil are the most commonly used immunosuppressive drugs for ocular inflammatory diseases, that mycophenolate is more expensive, and that comparative effectiveness data are limited, it is important to systematically investigate which is the better approach to guide clinicians as to which should be first line therapy.
Randomization provides the ideal solution to the indication-for-treatment bias that may occur when comparing alternative treatments but is not feasible to implement for every comparison. Also, confirmation of randomized clinical trial results with observational data can be useful, as outcomes tend to be better in prospective clinical trials. Robins and co-workers have developed a series of methods that can mitigate the problem of indication-for-treatment bias with observational data; of these, Marginal Structural Models are the easiest to implement.8,9 The premise of this method is to weight each observation by the inverse probability of being given the treatment in question based on the available covariates (clinical characteristics and treatment history). The weighting creates a population in which covariate and treatment history of alternative treatment groups is not associated with subsequent treatment. This method mimics a sequentially randomized trial and provides “real world” data that are important in interpreting comparisons outside clinical trials. A separate weighting procedure also accounts for censoring.10 Rubin previously summarized the value of this sort of methodology in the Journal, describing another method for accomplishing similar goals using observational data.11 Here, we apply the marginal structural models in order to compare the effectiveness of methotrexate versus mycophenolate mofetil for ocular inflammatory diseases.
Methods
The methods of the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study have been described previously,12 which also has reported results for the outcomes of methotrexate (MTX)13 and mycophenolate mofetil (MMF)14 separately using simple cohort analysis. The current analysis is undertaken to simulate a clinical trial as well as possible using observational data, applying the marginal structural models approach, so as to provide a comparison of relative benefits of these two treatments during a period time when both treatments were in use. The value of this kind of approach was described in the American Journal of Ophthalmology methodology series in a discussion of the propensity score method11 which assesses a patient’s probability of being treated versus control as a function of all relevant observed covariates. The marginal structural model method we use here8–10 accomplishes goals similar to the propensity score method, using biostatistical modeling of cohort data to weight the real longitudinal observations of patients treated with MTX or MMF by their probability of being given one treatment or another and of being censored during follow-up based on their pattern of covariates. Applying the weights to the patients longitudinally observed in the SITE Cohort Study creates a weighted population in which in which initial treatment and subsequent treatment changes are not associated with measured levels of prior covariates, so that initial treatment use and changes are unconfounded by measured covariates.8–10 These groups are then evaluated to assess time to resolution of ocular inflammation.
In brief, the cohort for this analysis consists of all patients with ocular inflammation, having sufficient data to carry out the analysis, who were seen at 4 ocular immunology and uveitis centers since the inception of the center. Information on all patients with noninfectious ocular inflammation in the parent study was collected using a common study protocol with quality control mechanisms. The available information for every eye of every patient at every visit was captured in a custom-built Microsoft Access database (Microsoft Corporation, Redmond, Washington, USA). Data relevant to this report included demographic information, ophthalmologic examination findings, presence or absence of systemic illnesses, ocular surgeries, and all medications in use at every clinic visit (including all use of corticosteroids and immunosuppressive drugs).
Only patients seen during the era in which MMF was used were included in this analysis (1998–2007). Patients were included only if they were at risk of the (favorable) event of interest: control of inflammation on prednisone ≤10 mg/day for ≥2 visits spanning ≥30 days. Only patients treated with single agent immunosuppression using MTX or MMF (plus corticosteroids) were included; person-time under combination immunosuppression was excluded. Due to the differing inclusion criteria for this comparative analysis and our prior simple cohort analyses of the outcomes of MTX and MMF,13–14 the sample sizes and person-time for this analysis differ from those reports.
Application of Marginal Structural Models to MTX vs. MMF The statistical approach involved three steps:
Step 1: Derive inverse probability weights from a model where treatment given (MMF vs MTX) is the outcome. We took into consideration all available covariates that might influence how a decision is made to treat ocular inflammation with either MTX or MMF. The approach modeled the extent to which the groups treated with MTX or MMF were different based on the covariate distributions. Application of weights derived from this model to the observational data created a weighted population un-confounded by the covariates. Although the study wasn’t a true randomized study, this approach addresses the methodological issue of indication-for-treatment to the extent that the covariate distribution captures the probability of going on one treatment or the other. (Randomization in a reasonably large study accomplishes this too, and also balances any unknown covariates). Covariates assessed included demographic characteristics (age, sex, race) and clinic site; systemic inflammatory disease status (presence of one of 25 immune-mediated diseases or not);6 Charlson Index of systemic morbidity;15 inflammatory activity & visual acuity at “baseline”; site of inflammation; time of evaluation - before or after 2000 (the point after which MMF began to be used more frequently than MTX in the cohort); history of prior ocular surgery; and the presence of ocular uveitic complications such as ocular hypertension, hypotony, band keratopathy, macular edema, epiretinal membrane, exudative retinal detachments, and presence of choroidal neovascular membranes. History of prior treatments (biologics, t-cell inhibitors, alkylators) also was included (although during the person-time analyzed all patients included were going onto single agent immunosuppressive therapy with MTX or MMF in addition to systemic corticosteroids, some had taken immunosuppressive treatments in the past). The model was used to derive weights proportional to the inverse of the probability of being assigned to and remaining on MMF or MTX. The method used stabilization of weights when appropriate to avoid undue influence of unusual or extreme values and to reduce the variance of the final estimates.8–10 Stabilization involves fitting a model for the treatment using as predictors prior treatment and baseline covariates included in the model for outcome (step 3, below), then multiplying the weights derived above (unstabilized) by the probability of observed treatment derived from this new treatment model.
Step 2: Derive weights from a model estimating the probability of censoring. This model used the same approach as in Model 1 with censoring as the outcome of interest. Applying weights derived in this step works similarly to balance the two treatment groups in the probability of being censored during follow-up. An approach similar to that described above was used to derive stabilized censoring weights.
Summarizing these steps, in the weighted population derived by applying the unstabilized weights from step 1 to the observational data, initial treatment and subsequent changes are not associated with measured levels of prior covariates, and so initial assignment and changes are unconfounded by measured covariates.8,10 In the weighted population derived by further applying weights from step 2, results will be as if no censoring had occurred; the weighting allows subjects censored at a given time to be represented for subsequent followup by subjects with similar measured histories through that time but not yet censored. We applied both treatment and censoring weights simultaneously.
Step 3: Apply weights to the final survival analyses evaluating time-to-corticosteroid-sparing success. First, we fit survival curves for success under MMF or MTX, using unstabilized weights. We then fit a proportional hazards model using the virtual populations generated by applying the weights derived from Models 1 and 2. Finally, we fit a proportional hazards model with time by treatment interactions to examine how the effect of MMF versus MTX varies over time, and a model with disease type by treatment interactions to examine whether or how the effect of treatment is modified by disease type.
Sensitivity analyses were performed using stabilized weights and using a general linear model with repeated measures instead of a proportional hazards model.
Results
Three hundred fifty two patients met inclusion criteria within the study period of interest i.e “monotherapy” with one agent or the other. Of these, 222 patients received MTX monotherapy and 132 patients MMF monotherapy for the indication of control of ocular inflammation and corticosteroid-sparing effect. Patient characteristics in both groups at baseline are given in Table 1. There were significant differences in age between the two groups, with MMF patients being on average 5 years older than MTX patients (Mean (SD) 46.49 years vs 41.26 years respectively, p 0.02). There were no important differences in gender, duration of inflammation, uni- vs. bilaterality, or history of prior immunosuppression between the groups. Consistent with the preferences of clinicians stated in a recent survey,16 the proportion of methotrexate-treated patients who had anterior uveitis was higher than the proportion of MMF-treated patients [80 patients on MTX (36%) vs 18 patients receiving MMF (14%); p= 0.0003]. Conversely, a smaller percentage of patients with posterior or panuveitis received MTX [55 patients (25%) than MMF (48 patients, 37%); p= 0.0003].
Table 1 –
Patient characteristics at outset of treatment with methotrexate or mycophenolate mofetil as the only immunosuppressive treatment, Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study, 1998–2007
| Variable | MMF | MTX | P-value[a] | |
|---|---|---|---|---|
| Number of Patients | 130 | 222 | ||
| Age (Years) | ||||
| Mean (SD) | 46.49 (16.68) | 41.26 (21.28) | 0.02 | |
| Median (Range) | 48.34 (10–83) | 42.11 (0–90) | ||
| Gender | 0.15 | |||
| Male | 52 (40.0%) | 72 (32.4%) | ||
| Female | 78 (60.0%) | 150 (67.6%) | ||
| Race | 0.46 | |||
| White | 91 (70.0%) | 166 (74.8%) | ||
| Black | 25 (19.2%) | 40 (18.0%) | ||
| Other | 14 (10.8%) | 16 (7.2%) | ||
| Anatomic uveitis diagnosis | 0.0003 | |||
| Anterior uveitis | 18 (13.8%) | 80 (36.0%) | ||
| Intermediate uveitis | 19 (14.6%) | 29 (13.1%) | ||
| Posterior or Panuveitis | 48 (36.9%) | 55 (24.8%) | ||
| Scleritis | 24 (18.5%) | 35 (15.8%) | ||
| Mucous Membrane | 10 (7.7%) | 16 (7.2%) | ||
| Other | 11 (8.5%) | 7 (3.2%) | ||
| Duration of Inflammation (Years) | Median (Range) | 2.05 (0–21) | 1.53 (0–24) | - |
| Bilateral | Yes | 97 (74.6%) | 162 (73.0%) | 0.74 |
| Prior any immunosuppression | Yes | 11 (8.5%) | 16 (7.2%) | 0.67 |
P-value for group comparisons. Continuous variables analyzed by general linear model. Categorical variables analyzed by χ2 test.
MMF - mycophenolate mofetil
MTX - methotrexate
Eye-specific characteristics are given in Table 2. There were 227 eyes on MMF monotherapy and 384 eyes on MTX monotherapy. Briefly, there were no major differences in initial visual acuity or clinically determined uveitis activity in either treatment group. Review of structural ocular complications showed that the eyes receiving MMF, were more likely to have macular edema [21 eyes (9.3%) on MMF vs. 18 eyes (4.7%) on MTX; p=0.03] and tended to be more likely to have an epiretinal membrane at baseline [26 eyes (11.5%) on MMF vs. 28 eyes (7.3%) on MTX; p=0.08], consistent with more posterior and panuveitis in the MMF group.
Table 2 –
Eye characteristics at outset of treatment with methotrexate or mycophenolate mofetil as the only immunosuppressive treatment, Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study, 1998–2007
| Variable | MMF | MTX | P-value[a] | |
|---|---|---|---|---|
| Number of Eyes | 227 | 384 | - | |
| Visual Acuity <20/50 | Yes | 163 (71.8%) | 252 (65.6%) | 0.11 |
| Visual Acuity <20/200 | Yes | 204 (89.9%) | 331 (86.2%) | 0.18 |
| Clinical uveitis activity | ||||
| Inactive | 146 (64.3%) | 237 (61.7%) | 0.37 | |
| Active | 52 (22.9%) | 105 (27.3%) | ||
| Slightly Active | 29 (12.8%) | 40 (10.4%) | ||
| Missing | 0 (0%) | 2 (0.5%) | ||
| Macular edema | Yes | 21 (9.3%) | 18 (4.7%) | 0.03 |
| Epiretinal membrane | Yes | 26 (11.5%) | 28 (7.3%) | 0.08 |
P-value for group comparisons. Continuous variables analyzed by general linear model. Categorical variables analyzed by chi-square.
MMF – mycophenolate mofetil
MTX - methotrexate
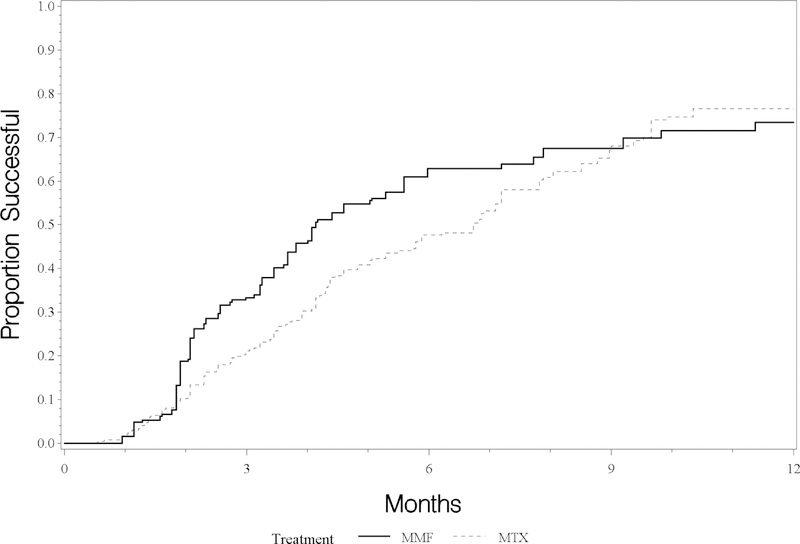
As seen in Figure 1, the weighted survival curves—adjusted for the factors differing between groups using the marginal structural model method (see methods)—appear to diverge after about two months, with an advantage for MMF, and then come back together by about nine months. The time-to-treatment success was significantly better with MMF than MTX, with a median time-to-treatment success with MMF was 8.2 months (5.3–12.8 months), vs 9.9 months (5.8–15.0 months) for MTX. Subsequently, the curves for both drugs become less steep, indicating diminishing (but non-zero) chances of treatment success following 6–9 months of therapy. We examined the changing pattern over time by adding time-by-treatment interaction terms to the proportional hazards model. The interaction term indicated that the hazard ratio may decline over time, consonant with the decreasing separation of the curves later on (Figure 1); this decline does not reach conventional levels of statistical significance. We also examined modification of the effect of treatment by disease type by including interaction terms; the p-value for interaction was 0.30, failing to provide strong evidence for such modification.
Figure:
Estimated time-to-control of ocular inflammation while taking prednisone 10 mg/day or less, by use of mycophenolate mofetil (MMF) or methotrexate (MTX)
Table 3 shows factors associated with corticosteroid sparing success with MTX and MMF, including only variables that were significant in crude models (not shown). Race, gender and use of prior calcineurin inhibitors were not associated with response to treatment. Age was associated with an increase in risk (Hazard ratio 1.009, 95% confidence interval 1.001–1.018; i.e., each additional year of age was associated with a 0.9% increase in the highest rate). MMF had a statistically significant overall 32% higher rate of corticosteroid sparing success as compared to MTX (Hazard ratio 0.68, 95% confidence interval 0.46–0.99), reflecting the shorter time-to-treatment success. Subjects with anterior uveitis had around 60% higher rate of corticosteroid sparing success compared to subjects with intermediate uveitis, posterior/panuveitis, MMP, scleritis and other forms of ocular inflammation. Associated systemic disease had a lower incidence of corticosteroid sparing success (Hazard ratio 0.60, 95% confidence interval 0.41–0.88). The median dose at which treatment success was observed was 12.5 mg/week in the MTX group and 1 gm twice daily in the MMF group.
Table 3 –
Factors associated with risk of treatment success
| Variables* | Hazard Ratio (HR) | 95% Confidence Interval on HR | ||
|---|---|---|---|---|
| Age (per year) | 1.009 | 1.001 | 1.018 | |
| White Race | 0.98 | 0.66 | 1.45 | |
| Male | 0.86 | 0.63 | 1.18 | |
| Prior calcineurin inhibitors | 0.90 | 0.48 | 1.68 | |
| Systemic Autoimmune disease | 0.60 | 0.41 | 0.88 | |
| Site of ocular inflammation | Anterior uveitis | 1.00 | ||
| Intermediate uveitis | 0.37 | 0.22 | 0.62 | |
| Posterior/panuveitis | 0.38 | 0.24 | 0.58 | |
| Scleritis | 0.39 | 0.21 | 0.70 | |
| Mucus membrane pemphigoid | 0.43 | 0.21 | 0.90 | |
| Other | 0.72 | 0.34 | 1.49 | |
| Baseline Calendar year <2000 | 1.086 | 0.99 | 1.20 | |
| MTX (vs MMF) | 0.68 | 0.46 | 0.99 | |
includes only variables “significant” in crude models
MMF – mycophenolate mofetil
MTX - methotrexate
Discussion
In this analysis, MMF demonstrated a shorter time-to-control of inflammation on prednisone≤10 mg/day as compared to MTX. The trajectory of the time-to-success curve suggests that MTX may eventually have a similar proportion with success. However, during the longer time-to-success higher doses of corticosteroids may have to be used and/or activity may be present, thereby suggesting an advantage for MMF for the average patient with ocular inflammation.
Brown et al conducted a survey of 11 uveitis experts from the American Uveitis Society to assess expert opinion on the relative effectiveness of MTX or MMF as an initial corticosteroid-sparing agent for the treatment of intermediate, posterior, and panuveitis. Eight of 11 experts (73%) believed MMF was more effective, with odds of treatment success for patients taking MMF 1.4-fold the odds of those taking MTX (95% Credible Interval 0.03–45.0)17 similar to our results. A preliminary randomized clinical trial compared the two drugs conducted in by Rathinam et al in South India,18 randomized subjects to receive either 25 mg oral MTX weekly (the typical maximum dose, as opposed to lower doses used for most patients in our study) or 2000mg oral MMF daily (the typical starting dose, the same as was used by the median patient in our study), and evaluated treatment success in a manner similar to the approach of our study. After 6 months, 69% percent of patients achieved treatment success with MTX and 47% with MMF, which was not significantly different (P = 0.09) but tended to favor methotrexate. The median time to achieve treatment success was 4.6 months for MTX and 4.1 months for MMF (p=0.44)—the latter similar to the result in our study, the former shorter than in our study.18 A Bayesian analysis combining the expert opinion survey results and the results of the trial showed the odds of treatment success with MMF compared to MTX 0.7 (95% Credible Interval 0.2–1.7).17 A Bayesian analysis uses Bayes’ Theorem to combine a prior distribution, sometimes derived from expert opinion, with data (and a model or likelihood) to obtain a posterior distribution, often summarized by a point estimate (posterior mean or median) and an interval estimate (credible interval).
Galor et al compared the relative effectiveness and side effect profiles of patients with uveitis that were treated with an antimetabolite as a first-line immunosuppressive agent from 1984 to 2006, in a single tertiary care uveitis clinic (one of the centers contributing to the SITE Cohort Study). The median time-to-treatment success, also defined as control of inflammation with ability to taper prednisone to 10mg or less daily, was 4.0, 4.8, and 6.5 months for the MMF, azathioprine, and MTX treatment groups, respectively (P = 0.02, log-rank test).19 Comparison of retention time, a measure of the duration of treatment with any given drug among 302 patients in a uveitis clinic in Portland, OR (not included in this analysis of SITE data) found that MTX was more effective and better tolerated than other immunosuppressive agents such as azathioprine, MMF and cyclosporine.20
Our results were within the credible interval on estimates from the Bayesian analysis, and were similar to the report of Galor et al (which included a minority of the same patients used in our analysis). Although the apparent differences between Rathinam’s18 pilot trial and our results might have occurred randomly, one might speculate that the two-fold higher dose of methotrexate in Rathinam’s18 preliminary trial than what was used at the time of success in our study (whereas MMF doses were similar) could have contributed to a different pattern of results. Our prior publication found no significant difference in corticosteroid sparing with either oral or subcutaneous route of MTX administration,13 so it is unlikely that differing routes of administration explain differences. If forthcoming clinical trial data suggest better results with methotrexate than we observed, one potential explanation is that starting with a higher dose of methotrexate is more effective. It also is possible that starting with a higher dose of MMF also would improve time-to-success.
Strengths of our analysis include a large sample size and subsequent favorable statistical power, standardized data collection and reporting21 and a large array of demographic and clinical characteristics available for use in inverse probability weighting. While designed to provide a fair comparison between two treatment modalities, the method has some limitations. Prior observational comparisons have been difficult because disease characteristics predictive of outcome may inform clinicians’ choices regarding which agent to use. The available covariates (clinical characteristics and treatment history) therefore must convey the information that was used in treatment selection; if not, weighting or other methods will incompletely adjust for these factors. The method cannot adjust for unknown or unmeasured confounders, which tend to be balanced by randomization but are not balanced by our analytic weighting methods.10 Thus, avoidance of indication-for-treatment bias could have been incomplete, which might have biased our results if there were large unknown differences. The same limitation applies to our adjustments for censoring. Our models for the initial treatment assignment, change, and censoring must faithfully represent the associations of covariates with those processes. None of these assumptions is guaranteed. It is unlikely that corticosteroid tapering systematically differed by treatment group, which would be needed to cause a bias. Also, if there was such a difference, it would need to be very large to impact our outcome requiring corticosteroid-sparing to be sustained for atleast 30 days. The clinics in our study were tertiary uveitis clinics in academic institutions, and hence the results are less generalizable to the general ophthalmology setting, although a large proportion of immunosuppressive therapy for uveitis is administered in subspecialty clinics to which our results should be generalizable. Another potential concern is that our approach uses the experience of uncensored subjects to represent the experience of uncensored subjects after censoring. We examined the extent of this issue, by looking at the distribution of censoring weights for each subject at time of their last visit. For unstabilized weights, the mean was 1.14 and the largest was 1.74, implying that each subject was represented by themselves and 0.74 or lesser of another subject in terms of censoring, which is within acceptable limits for such analyses. Lastly, although anatomic site of inflammation was not statistically different between treatment groups, specific uveitic entities might respond differently to treatment and would not have been uncovered by our analysis.
In conclusion, our marginal statistical model-based comparison of MTX vs. MMF for ocular inflammatory diseases suggests that both MMF and MTX had similar corticosteroid sparing effect at 9 months, however MMF more rapidly achieves corticosteroid-sparing effect than does MTX which potentially avoids the risk factors associated with corticosteroid use and active ocular inflammation for 9 months. Starting with a relatively low dose of methotrexate, consistent with expert panel guidelines,1 might have contributed to longer time to success with that agent. Given that the safety profile of MMF14 is no worse than that of MTX,13 and the greater rapidity of MMF to accomplish corticosteroid-sparing goals (control of uveitis with a dose of prednisone that is relatively safe for the medium term) MMF may be preferred if there is no specific indication for MTX in order to minimize corticosteroid-induced side effects and gain stable control of uveitis sooner to avoid uveitis-induced complications. However, it is possible that starting with an initially higher dose of methotrexate as in Rathinam’s18 preliminary trial would improve outcomes with methotrexate, mitigating the apparent advantage of MMF in our study; we look forward to more data on this subject. The marginal structural model method and similar methods like G-estimation22–23 and propensity scores 11potentially are useful in comparing treatments based on observational data, which may supplement information from randomized trials or provide comparative data when clinical trials are not available. These methods of analyzing observational data provide a complementary perspective due to the different strengths and weakness as compared to a clinical trial and generate questions to be studied and explored in the future.
Supplementary Material
Highlights.
Methotrexate and mycophenolate mofetil have shown efficacy and safety when used for ocular inflammation. A simulated head to head trial shows that mycophenolate may have shorter time to resolution of inflammation compared to methotrexate.
Acknowledgements/Disclosure
a. Funding Support
This study was supported primarily by National Eye Institute Grant EY014943 (JHK). Additional support was provided by Research to Prevent Blindness, the Paul and Evanina Mackall Foundation, and the Lois Pope Life Foundation. JHK was an RPB James S Adams Special Scholar Award recipient, JET was an RPB Harrington Special Scholar Award recipient, and DAJ and JTR were Research to Prevent Blindness Senior Scientific Investigator Award recipients during the course of the study. GAL-C and RBN were previously supported by and HNS continues to be supported by intramural funds of the National Eye Institute. EBS receives support from the Department of Veterans’ Affairs. None of the sponsors had any role in the design and conduct of the report; collection, management, analysis, and interpretation of the data; or in the preparation, review, and approval of this manuscript.
Footnotes
b. Financial Disclosure(s)
The author(s) have made the following disclosure(s):
Sapna Gangaputra: (grant recipient) Research to Prevent Blindness, USAID
C. Stephen Foster: (equity owner) Eyegate, (consultant, lecturer) Allergan, Bausch & Lomb; (consultant) Sirion; (lecturer) Alcon, Inspire, Ista, Centocor.
James Rosenbaum: (consultant) Abbvie; UCB, Gilead, Regeneron, Eyevensys; (grant recipient) Pfizer; (equity owner) Novartis; (royalties) UpToDate
Jennifer Thorne: (consultant) AbbVie, Gilead, Santen; (grant recipient) National Eye Institute, Allergan.
John H. Kempen: (consultant) Clearside, Gilead, and Santen; (grant recipient) National Eye Institute, Sight for Souls, Christian Blind Mission International, EyeGate Pharma.
All of the other authors have no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 2000; 130:492–513. [DOI] [PubMed] [Google Scholar]
- 2.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group, Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Louis TA, Sugar EA, Thorne JE. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011. October;118(10):1916–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group, Kempen JH, Altaweel MM, Drye LT, Holbrook JT, Jabs DA, Sugar EA, Thorne JE. Benefits of Systemic Anti-inflammatory Therapy versus Fluocinolone Acetonide Intraocular Implant for Intermediate Uveitis, Posterior Uveitis, and Panuveitis: Fifty-four-Month Results of the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study. Ophthalmology. 2015. October;122(10):1967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Writing Committee for the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study Research Group, Kempen JH, Altaweel MM, Holbrook JT, Sugar EA, Thorne JE, Jabs DA. Association Between Long-Lasting Intravitreous Fluocinolone Acetonide Implant vs Systemic Anti-inflammatory Therapy and Visual Acuity at 7 Years Among Patients With Intermediate, Posterior, or Panuveitis. JAMA. 2017. May 16;317(19):1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group, Kempen JH, Altaweel MM, Drye LT, Holbrook JT, Jabs DA, Sugar EA, Thorne JE. Benefits of Systemic Anti-inflammatory Therapy versus Fluocinolone Acetonide Intraocular Implant for Intermediate Uveitis, Posterior Uveitis, and Panuveitis: Fifty-four-Month Results of the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study. Ophthalmology. 2015. October;122(10):1967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempen JH, Daniel E, Dunn JP, Foster CS, Gangaputra S, Hanish A, Helzlsouer KJ, Jabs DA, Kaçmaz RO, Levy-Clarke GA, Liesegang TL, Newcomb CW, Nussenblatt RB, Pujari SS, Rosenbaum JT, Suhler EB, Thorne JE. Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study. BMJ. 2009. July 3;339: b2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frick KD, Drye LT, Kempen JH, Dunn JP, Holland GN, Latkany P, Rao NA, Sen HN, Sugar EA, Thorne JE, Wang RC, Holbrook JT; Multicenter Uveitis Steroid Treatment-MUST Trial Research Group. Associations among visual acuity and vision- and health-related quality of life among patients in the multicenter uveitis steroid treatment trial. Invest Ophthalmol Vis Sci. 2012. March 9;53(3):1169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000. September;11(5):550–60. [DOI] [PubMed] [Google Scholar]
- 9.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000. September;11(5):561–70 [DOI] [PubMed] [Google Scholar]
- 10.Joffe MM, Pistilli M, Kempen JH. Marginal structural models for comparing alternative treatment strategies in ophthalmology using observational data. Ophthalmic Epidemiol. 2013. August;20(4):197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin Donald B. Propensity Score Methods. American Journal of Ophthalmology, Volume 149, Issue 1, 7–9 [DOI] [PubMed] [Google Scholar]
- 12.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol 2008;15:47–55. [DOI] [PubMed] [Google Scholar]
- 13.Gangaputra S, Newcomb CW, Liesegang TL, Kaçmaz RO, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Kempen JH; Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009. November;116(11):2188–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel E, Thorne JE, Newcomb CW, Pujari SS, Kaçmaz RO, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Suhler EB, Foster CS, Jabs DA, Kempen JH. Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol. 2010. March;149(3):423–32.e1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83 [DOI] [PubMed] [Google Scholar]
- 16.Esterberg E, Acharya NR. Corticosteroid-sparing therapy: practice patterns among uveitis specialists. J Ophthalmic Inflamm Infect. 2012;2(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Browne EN, Rathinam SR, Kanakath A, Thundikandy R, Babu M, Lietman TM, Acharya NR. A Bayesian Analysis of a Randomized Clinical Trial Comparing Antimetabolite Therapies for Non-Infectious Uveitis. Ophthalmic Epidemiol. 2017. February;24(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rathinam SR, Babu M, Thundikandy R, Kanakath A, Nardone N, Esterberg E, Lee SM, Enanoria WT, Porco TC, Browne EN, Weinrib R, Acharya NR. A randomized clinical trial comparing methotrexate and mycophenolate mofetil for noninfectious uveitis. Ophthalmology. 2014. October;121(10):1863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galor A, Jabs DA, Leder HA, Kedhar SR, Dunn JP, Peters GB 3rd, Thorne JE. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology. 2008. October;115(10):1826–32 [DOI] [PubMed] [Google Scholar]
- 20.Baker KB, Spurrier NJ, Watkins AS, Smith JR, Rosenbaum JT. Retention time for corticosteroid-sparing systemic immunosuppressive agents in patients with inflammatory eye disease. Br J Ophthalmol. 2006. December;90(12):1481–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005. September;140(3):509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robins J The control of confounding by intermediate variables. Stat Med 1989;8:679–701. [DOI] [PubMed] [Google Scholar]
- 23. Robins JM, Blevins D, Ritter G, Wulfsohn M. G-estimation of the effect of prophylaxis therapy for Pneumocystis carinii pneumonia on the survival of AIDS patients. Epidemiology 1992;3:319–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.