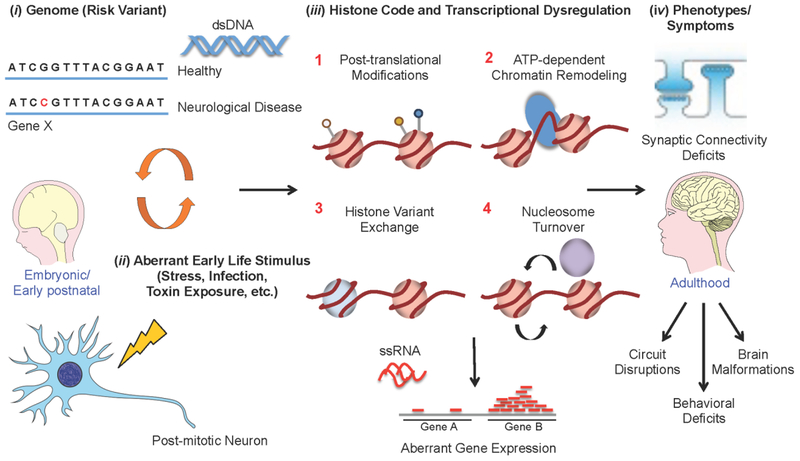
Fig. 1: Histone dysregulation in neurological disease.
Aberrant alterations of histone PTMs and chromatin structures are thought to result in disrupted patterns of neurodevelopment and subsequent synaptic/behavioral abnormalities in adulthood. These disorders are precipitated by a combination of (i) genetic risk variants (i.e., DNA mutations) and (ii) environmental risk factors (e.g., early life stress, exposure to toxins, infections, etc.), the latter of which can result in (iii) profound alterations in gene expression through aberrant regulation of histones (e.g., 1–PTMs, 2–chromatin remodeling, 3–histone variant exchange and 4–nucleosome turnover) and other “epigenetic” phenomena. (iv) These molecular processes may then be responsible for many of the physiological and behavioral phenotypes observed in neurological disease during adulthood. Much work is still needed, however, to truly be able to causally link such “epigenetic” mechanisms to neurological illness, a process that will require both the development of novel methodologies (e.g., in vivo protein trans-splicing, etc.) and a concerted effort by the field to assess molecular outcomes within a brain specific framework.