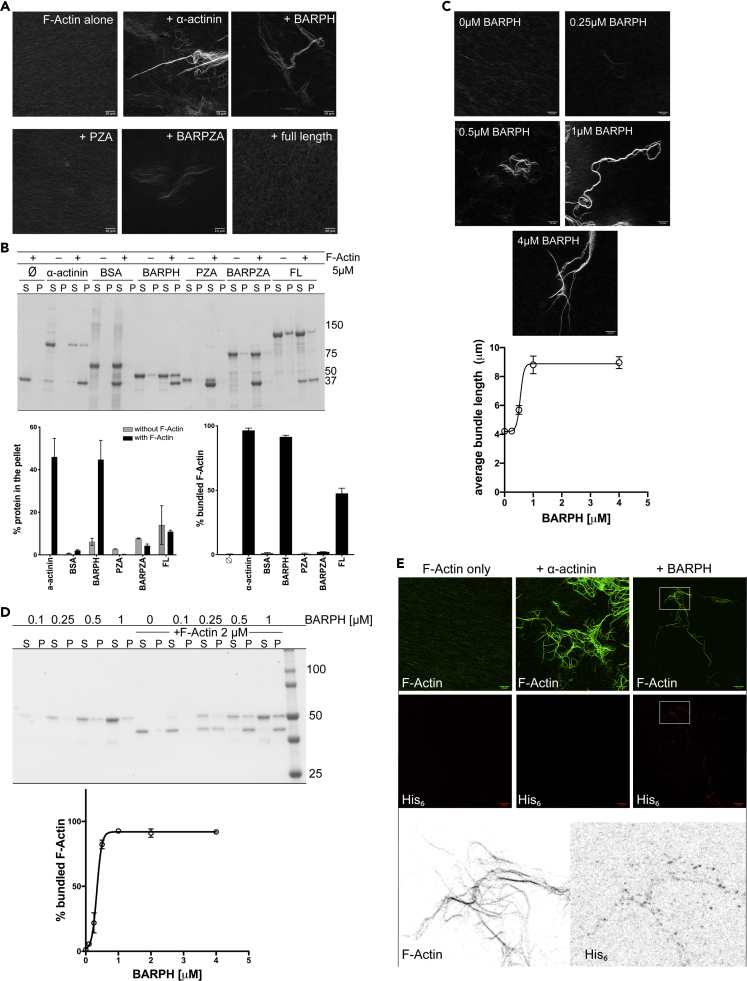
Figure 5.
ASAP1 Bundles Actin Filaments In Vitro through Its N-BAR Domain and the C Terminus of ASAP1 Negatively Modulates the Bundling Activity
(A) Effect of ASAP1 on bundling of F-actin assessed by fluorescence microscopy. Rabbit muscle F-actin (3.3 μM) alone or with ASAP1, the indicated truncations of ASAP1, or α-actinin (positive control) (3.3 μM) were mixed in F-actin buffer and spotted on poly-L-lysine-coated coverslips. After 30 min, coverslips were fixed and stained with rhodamine phalloidin. Images were taken on Leica SP8 confocal microscope using 63× oil objective. Representative maximum intensity projection images are shown, scale bar, 10 μm. Data are representative of 5 independent experiments.
(B) Effect of ASAP1 on bundling of F-actin assessed by a low-speed co-sedimentation assay. F-actin (5 μM) alone, ASAP1 or ASAP1 truncations or α-actinin alone (2 μM), or F-actin with the indicated proteins were subjected to low-speed co-sedimentation assay. Equal volumes of the supernatant (S) and resuspended pellet (P) were resolved on gradient SDS-PAGE and stained with GelCode Blue. A gel representative of three experiments is shown in the upper panel. In the lower left panel, the percentage of the indicated proteins that sedimented in the absence and presence of F-actin, calculated from three experiments, is shown. In the right lower panel, the percentage of F-actin that sedimented in the presence of the indicated proteins is shown. The means ± SEM from three experiments are presented. See also Figures S4A–S4C.
(C) Effect of BAR-PH and length of F-actin bundles. F-actin (1 μM) was incubated with the indicated concentrations of BAR-PH and subjected to IF-based bundling assay as in (A). Filament length was approximated using the ridge plugin in Fiji/ImageJ. Results are representative of three independent experiments. Results are summarized as the mean ± SEM, Scale bar, 10 μm.
(D) Bundling efficiency of BAR-PH. The percentage of F-actin bundled at different concentrations of BAR-PH was determined by the sedimentation assay. Increasing concentrations of BAR-PH (0.1–4 μM) were titrated into a solution containing a fixed concentration of F-actin (2 μM) and incubated for 30 min at 23°C. Actin bundles were separated from free filaments by centrifugation at 14,000 × g for 30 min. A representative gel is shown in the upper panel. A summary of the combined results of three experiments is shown in the lower panel. The mean % bundled F-actin ± SEM at each BAR-PH concentration is presented.
(E) Incorporation of BAR-PH into actin bundles. Colocalization of BAR-PH with actin bundles was assessed by immunofluorescence. F-Actin alone (1 μM), with α-actinin (1 μM, tagless), as a positive actin-bundling control, or with ASAP1 BAR-PH (1 μM, His6-tagged) was incubated as in (A) and double-stained for F-actin (phalloidin Alexa Fluor 488) and His6-tag (goat anti-mouse Alexa Fluor 594) and analyzed by microscopy as in (A). A single Z-slice for each condition is shown, brightness and contrast are enhanced equally for presentation purposes, scale bar, 10 μm. As α-actinin does not have a His6 tag, there was no signal in the His6 channel under this condition. Areas shown in white rectangles were enlarged with LUTs inverted to reveal puncta.