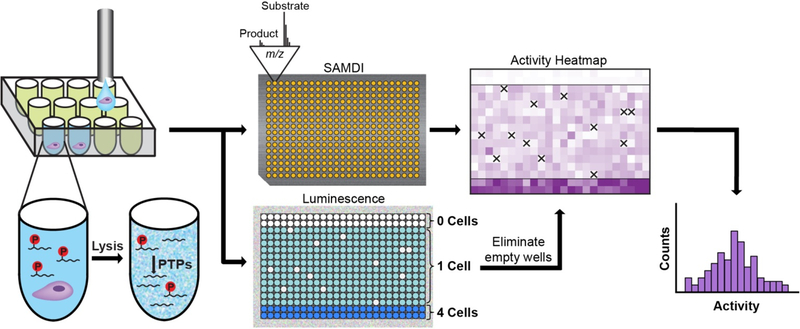
Figure 1.
Overview of the single-cell SAMDI assay. A suspension of individual cells, prepared in a buffer containing HRP, is sorted into a low-volume, 384-well plate by flow cytometry. Each well already contains lysis buffer and a phosphopeptide substrate (Ac-IpYERC-NH2). Once deposited, the cells are lysed, and active PTP enzymes can act on the substrate. Following an incubation period, the contents of the well plate are split into two pools. One pool is transferred onto an array of 384 gold islands—each presenting a maleimide-functionalized monolayer—to immobilize both substrate and product present in solution. The array is analyzed using SAMDI mass spectrometry, which quantitates PTP activity in each well by reporting the amount of substrate and product and therefore the yield. To the second pool, a luminescent HRP substrate is added, and the resulting luminescence signal is used to eliminate any wells that did not receive a cell due to error by flow cytometry (which are indicated by the ‘X’s in the heatmap). SAMDI measurements from the remaining wells reveal the distribution of single-cell activities.