Abstract
Rabies is a zoonotic disease that still causes 59,000 human deaths each year, and rabies vaccine is the most effective way to control the disease. Our previous studies suggested that the maturation of DC plays an important role in enhancing the immunogenicity of rabies vaccine. Flt3L has been reported to own the ability to accelerate the DC maturation, therefore, in this study, a recombinant rabies virus expressing mouse Flt3L, designated as LBNSE-Flt3L, was constructed, and its immunogenicity was characterized. It was found that LBNSE-Flt3L could enhance the maturation of DC both in vitro and in vivo, and significantly more TFH cells and Germinal Center B (GC B) cells were generated in mice immunized with LBNSE-Flt3L than those immunized with the parent virus LBNSE. Consequently, expressing of Flt3L could elevate the level of virus-neutralizing antibodies (VNA) in immunized mice which provides a better protection from a lethal rabies virus challenge. Taken together, our study extends the potential of Flt3L as a good adjuvant to develop novel rabies vaccine by enhancing the VNA production through activating the DC–TFH–GC B axis in immunized mice.
Keywords: Rabies, Vaccine, FMS-like tyrosine kinase 3 ligand (Flt3L), Dendritic cell (DC), Follicular helper T cell (TFH cell), Germinal center B cell
Introduction
Rabies is a zoonotic disease that causes 59,000 death every year (WHO 2013), which is still a huge threat to the public health, especially in the developing countries such as India and China (Okonko et al.2010; Acharya et al.2012). A large number of cases among wild animals are reported each year, even in some developed countries which are free of human rabies (Scotch et al.2009; Adedeji et al.2010). Its pathogen, rabies virus, retrogrades through the nerve from wound to central nerve system and cause disease in brain (Murphy and Bauer 1974; Alizadeh et al.2015). Rabies is fatal once the clinical signs appear, however, the disease is 100% preventable with appropriate vaccination (Ayres et al.2006; Abelaridder 2015). Millions of people receive rabies vaccination globally, which saves more than 250,000 people from dying of rabies every year (Wunner and Briggs 2010). According to the report from WHO, human rabies cases could be possibly reduced through a combination of interventions involving mass dog vaccination, improved access to post-exposure prophylaxis (PEP), increased surveillance and raising public awareness (Dodet 2010). It has been suggested that human rabies and rabies virus transmission would be efficiently reduced once the vaccination coverage of canine population is higher than 70% (Hu et al.2009). Therefore, it is necessary to develop efficacious rabies vaccine for a better rabies control.
Fms-like tyrosine kinase 3 ligand (Flt3L) is a hematopoietic growth factor with well-established functions in hematopoietic cell development, such as promoting lymphoid lineage commitment (Adolfsson et al.2001; Ge et al.2013) and driving dendritic cell (DC) development (Waskow et al.2008). Flt3L and its receptor (Flt3, FLK2) regulate the mobilization of preDC from the blood to give rise to DC in lymphoid organs and tissue-resident DC (Anandasabapathy et al.2014). Previous study demonstrated that protective immunity induced by protein vaccines is controlled by Flt3L-dependent, LN-resident DC and Flt3L-independent pathways can mediate infection-induced expansion of DC and T cell priming (Dupont et al.2015).
Mature DCs are professional antigen-presenting cells (APCs) (Mathers and Larregina 2006) that can induce T and B cell activation, thereby linking innate and adaptive immune responses (Garg et al.2012). DC activation has been demonstrated to be a good strategy to enhance the immunogenicity of RABV by expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-15 in our previous study (Chen et al.2017; Wen et al.2011; Zhou et al.2013, 2015).
Therefore, due to the function of Flt3L on enhancing DC maturation, an rRABV expressing Flt3L was constructed to further characterize the role of DC activation that plays in the immunogenicity of rabies vaccine, and it was found that expression of Flt3L could enhance the VNA production in immunized mice through enhancing the DC activation and generation of Follicular helper T (TFH) and Germinal Center B (GC B) cells. Our study suggests that Flt3L is a potential adjuvant to improve humoral immune responses to live attenuated rabies vaccine.
Materials and Methods
Cells, Antibodies, Viruses and Animals
BSR cells, a clone derived from BHK-21 cells, were cultured in Dulbecco’s modified Eagle’s medium (Mediatech, Manassas, VA, USA) containing 10% fetal bovine serum (FBS; Gibco). LBNSE is an rRABV that derived from the SAD-B19 strain (Conzelmann et al.1990; Rasalingam et al.2005) by making mutations at amino acid positions 194 and 333 of G protein (Wen et al.2011). Propagation of rRABV strains was performed in BSR cells. The challenge virus strain CVS-24 was propagated in the brains of 5–6 days old suckling mice. Fluorescein isothiocyanate (FITC)-conjugated antibodies against the RABV N protein were purchased from FujiRab (Melvin, PA). Antibodies used for flow cytometric analysis, including FITC anti-mouse CD11c (clone N418), FITC anti-mouse CD4 (clone GK1.5), FITC anti-mouse/human CD45R/B220 (clone RA3-6B2), PE anti-mouse CD86 (clone GL-1), PE anti-mouse CD279 (PD-1) (clone RMP1-30), PE/Cy7 anti-mouse I-A/I-E (MHC-II) (clone M5/114.15.2), APC anti-mouse CD185 (CXCR5) (clone L138D7), APC anti-mouse CD138 (Syndecan-1) (clone 281-2), and Alexa Fluor 647 anti-mouse/human GL7 (clone GL7), were all purchased from BioLegend (San Diego, CA), while PE Anti-Mouse CD95 (APO-1/Fas) (clone 15A7) was purchased from eBioscience (San Diego, CA). Recombinant mouse GM-CSF and IL-4 were purchased from Novoprotein Scientific, Inc. (Shanghai, China). 6–8 weeks old female BALB/c mice and ICR mice were purchased from the Center for Disease Control and Prevention of Hubei Province, China.
Construction and Rescue of Recombinant Virus
The mouse Flt3L gene was amplified from total RNA extracted from mouse spleen using the SuperScript III One-Step reverse transcription (RT)-PCR system with Platinum Taq DNA polymerase (Invitrogen Life Technology, Carlsbad, CA, USA). The primers used for Flt3L gene amplification: (forward primer: 5′-TTGCGTACGAACATGACAGTGCTGGCGCCAGC-3′ and reverse primer: 5′-CTAGCTAGCTAGCTAGGGATGGGAGGGGAGG-3′). Construction and virus rescue strategy of rRABV LBNSE-Flt3L infectious clone was followed as described before (Schnell et al. 1994; Wu et al.2014). Briefly, PCR production (Flt3L gene) and LBNSE infectious clone were both digested with BsiWI and NheI (New England BioLabs, Ipswich, MA, USA), and after recycling, the Flt3L gene was inserted between G and L genes of LBNSE by ligation to finish the construction of LBNSE-Flt3L infectious clone. For the rescue of LBNSE-Flt3L, BSR cells were transfected with 2.0 µg of LBNSE-Flt3L infectious clone, 0.5 µg of pH-N, 0.25 µg of pH-P, 0.15 µg of pH-G, and 0.1 µg of pH-L using the SuperFect transfection reagent (Qiagen) according to the manufacturer’s protocol. Then BSR cells were incubated at 37 °C for 4 days, and the supernatant was removed and fresh medium with 2% FBS was added to each well for further incubation. After 7 days of incubation, supernatant was collected to examine the presence of rescued virus using FITC-conjugated anti-RABV N Antibodies.
Virus Titration and Growth Curve Assay
The rRABVs were titrated via direct fluorescent antibody assays in BSR cells as previously described (Zhao et al.2009). Briefly, 100 µL serial 10-fold dilutions of the virus was incubated with BSR cells in 96-well plates at 37 °C for 48 h, and then direct fluorescent antibody assay was performed to test the RABV in each well. Viral titers were calculated as fluorescent focus units per milliliter. All titrations were carried out in quadruplicate. For the multi-step or one-step growth curve, BSR cells were seeded in a 12-well plate and infected with different rRABVs at a multiplicity of infection (MOI) of 0.01 or 5, respectively. After incubating for 1 h at 37 °C, the supernatant was removed and fresh medium with 2% FBS was added to each well. Samples were collected each day for 5 days and stored at − 80 °C. The growth curves were depicted according to the virus titers at different time points.
Virus-Neutralizing Antibody (VNA) Test
Blood samples were collected and the sera were isolated weekly for VNA test using the fluorescent antibody virus neutralization (FAVN) assay described previously (Tian et al.2015). Briefly, 50 µL of serial threefold dilutions of test or standard serum sample, obtained from the National Institute for Biological Standards and Control (Herts, UK), was prepared in 96-well microplates of 150 µL volumes. Each sample was added into four duplicated wells. A 50 µL volume containing 50–200 FFU of CVS-11 was added into each well and incubated at 37 °C for 1 h in an incubator with 5% CO2. Then 50 µL of BSR cells suspension, containing 2 × 104 of cells, was added into each well and incubated at 34 °C with 5% CO2 for 72 h. The plates were then fixed with 80% of ice cold acetone at − 20 °C for 30 min and air-dried. Cells were stained with FITC-conjugated anti-RABV N antibodies for 45 min at 37 °C and washed three times with PBS. The results were observed under an Olympus IX51 fluorescence microscope, and the VNA titers were expressed in international units per milliliter (IU/mL) based on comparisons with the titer of the reference serum sample included in each test.
Isolation of Bone Marrow-Derived DCs
Bone marrow-derived DCs were isolated as described previously (Lutz et al.1999; Gilboa 2007). Briefly, 6–8 weeks old BALB/c mice were euthanized and the femur was separated. Then bone marrow was collected and seeded in 6 well plates at the density of 2 × 105 cells/mL, and the cells were cultured in DC medium (RPMI 1640 supplemented with 10% FBS, 20 ng/mL recombinant mouse GM–CSF and 10 ng/mL recombinant mouse IL-4). At 1, 3, and 5 days post cultivation, half of the medium was replaced with fresh DC medium. At 7 days post cultivation, the cells were collected and cultured in 12 well plates (106 cells/mL), and the DCs would be used for the study 2 days later.
Flow Cytometry
Lymph nodes, bone marrows, and peripheral blood samples were collected from different rRABVs immunized mice at each tested time points post immunization for flow cytometric analysis. The collected lymph nodes and bone marrows were homogenized and filtered through a 40 µm nylon filter, then washed with PBS and resuspended as single cell suspensions with 0.2% BSA; for the blood samples, the red blood cells were lysed with ACK lysis buffer (BioSource International, Inc., Camarillo, CA) for 1 min at room temperature. Single-cell suspensions (106 cells/mL) were prepared and stained in 0.2% BSA with fluorescence-conjugated antibodies for 30 min at 4 °C in the dark. After incubation, the cells were subsequently washed twice with PBS and fixed in 4% paraformaldehyde for 30 min. The samples were performed through a BD LSR-II flow cytometer, and data collection and analysis were performed by BD FACS-Diva software (BD Pharmingen) and FlowJo software (TreeStar, San Carlos, CA).
Mice Immunization and RABV Challenge Experiment
Groups of female 6–8 weeks old ICR mice were immunized with 106 FFU of each rRABV in a volume of 100 μL or mock immunized with the same volume of DMEM by intramuscular (i.m.) route. At 21 days post immunization (dpi), mice were challenged with 50% mouse intracerebral lethal dose 50 (MICLD50) of CVS-24 in a volume of 40 μL by the intracerebral (i.c.) route and observed daily for 3 weeks.
Statistical Analysis
All data were analyzed by GraphPad Prism 6.0 software (La Jolla, CA). Kaplan–Meier survival curves were analyzed by the log rank test; the other data were determined by one-way ANOVA. For all tests, the following notations were used to indicate significant differences between different groups: *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Construction and Characterization of the rRABV Expressing Flt3L
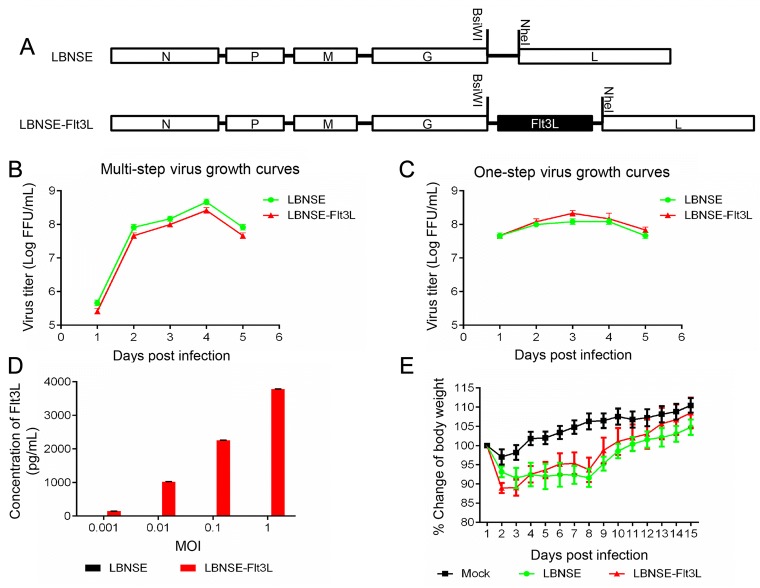
Flt3L was cloned and inserted into the genome of a RABV vaccine strain LBNSE which derived from SAD-B19 with two mutations in the glycoprotein (G), between G and L gene by using two restriction enzymes BsiW I and Nhe I as shown in Fig. 1A and the rRABV expressing Flt3L was designated as LBNSE-Flt3L. The rRABV LBNSE-Flt3L was rescued as described previously, and the multi-step (MOI = 0.01) and one-step (MOI = 5) growth kinetics of the rRABVs on BSR cells were depicted. As shown in Fig. 1B and 1C, the growth curves of LBNSE-Flt3L were similar as those of parent virus LBNSE, and no significant difference was found between the viral titers at each time points, indicating that the insertion of Flt3L did not affect viral replication in BSR cells. Next, to determine whether the Flt3L was expressed as expected, BSR cells were infected with LBNSE-Flt3L or LBNSE at different MOIs, and the supernatant was collected for detection of Flt3L by ELISA. As shown in Fig. 1D, BSR cells infected with LBNSE-Flt3L could produce Flt3L in a dose dependent manner, while the expression of Flt3L in LBNSE infected BSR cells was under detectable level. Moreover, to investigate whether the expression of Flt3L would affect the viral pathogenicity, groups of 10 mice were infected with 107 FFU of LBNSE, LBNSE-Flt3L or mock infected with the same volume of DMEM through i.c. route and the body weights were recorded daily for 2 weeks. No clinical symptoms were observed in all mice, and a slight increase on body-weight changes of mice infected with LBNSE-Flt3L than those infected with parent virus LBNSE was observed, although the change was statistically not significant (Fig. 1E), suggesting that expression of Flt3L could slightly attenuate the viral pathogenicity in mice.
Fig. 1.
Construction and characterization of rRABV expressing IL-15 in vitro and in vivo. A Schematic diagram for construction of LBNSE-Flt3L. The parent vector pLBNSE was constructed based on SAD-B19 strain by deleting the pseudogene between the G and L genes where BsiwI and NheI restriction enzyme sites were introduced. N, P, M, G and L represented the nucleoprotein, phosphoprotein, matrix, glycoprotein, and polymerase genes of RABV, respectively. Multi-step (B) and One-step (C) virus growth curves were determined on BSR cells. Cells were infected with either LBNSE or LBNSE-Flt3L at a multiplicity of infection (MOI) of 0.01 or 5, respectively, and the culture supernatants were harvested at 1, 2, 3, 4 and 5 dpi for viral titration. The virus growth curves were drawn according to the viral titers measured at each time point. Data are presented as the mean ± SD (n = 3). D Expression of Flt3L was detected in infected BSR cells by ELISA. BSR cells were infected with either the LBNSE or LBNSE-Flt3L at the MOI = 0.001, 0.01, 0.1, or 1, and the culture supernatants were harvested at 24 h post infection for ELISA assay. Data are presented as the mean ± SD (n = 3). E Body weight change curves of mice infected with different rRABVs. Six-week-old female ICR mice (n = 10) were infected by the i.c. route with 1 × 107 FFU of LBNSE or LBNSE-Flt3L, or with mock infected with the same volume of DMEM, and the body weights were monitored daily for 2 weeks.
In Vitro Activation of DC After Incubation with Different rRABVs
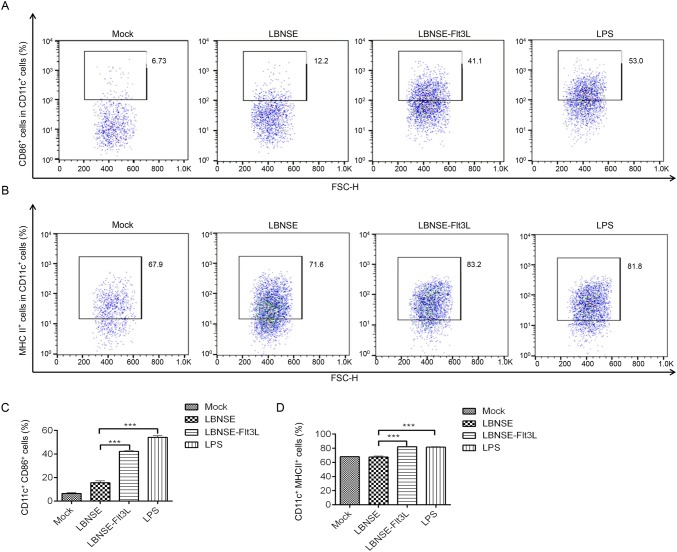
To investigate whether LBNSE-Flt3L could enhance the activation of DC, bone marrow-derived DC was prepared and incubated with LBNSE or LBNSE-Flt3L, and LPS and DMEM were employed as the positive and negative control, respectively. The activated DCs (CD11c+ CD86+, or CD11c+ MHC II+) were detected by flow cytometry, and the representative flow cytometric plots for activated DC, CD11c+CD86+ and CD11c+MHC II+, were shown in Fig. 2A and 2B respectively. As shown in Fig. 2C and 2D, significantly more CD86+ (Fig. 2C) or MHC II+ (Fig. 2D) cells in CD11c+ cells were detected in LBNSE-Flt3L infected or LPS incubated cells than those incubated with LBNSE. The results revealed that expression of Flt3L could improve DC activation.
Fig. 2.
In vitro activation of bone marrow-derived DC after infection with different rRABVs. Bone marrow cells were harvested from 6 to 8 weeks old female BALB/c mice, and DCs precursors were cultured with GM–CSF. LPS was used as positive control. A Representative flow cytometric plots for CD11c+CD86+ (A) and CD11c+MHC II+ (B) DC. C Percentages of CD11c+CD86+ activated DC after infection with different rRABVs. D CD11c+MHC II+ activated DC after infection with different rRABVs. Data were the means from three independent experiments (***P < 0.001).
Activation of DC in Mice Immunized with Different rRABVs
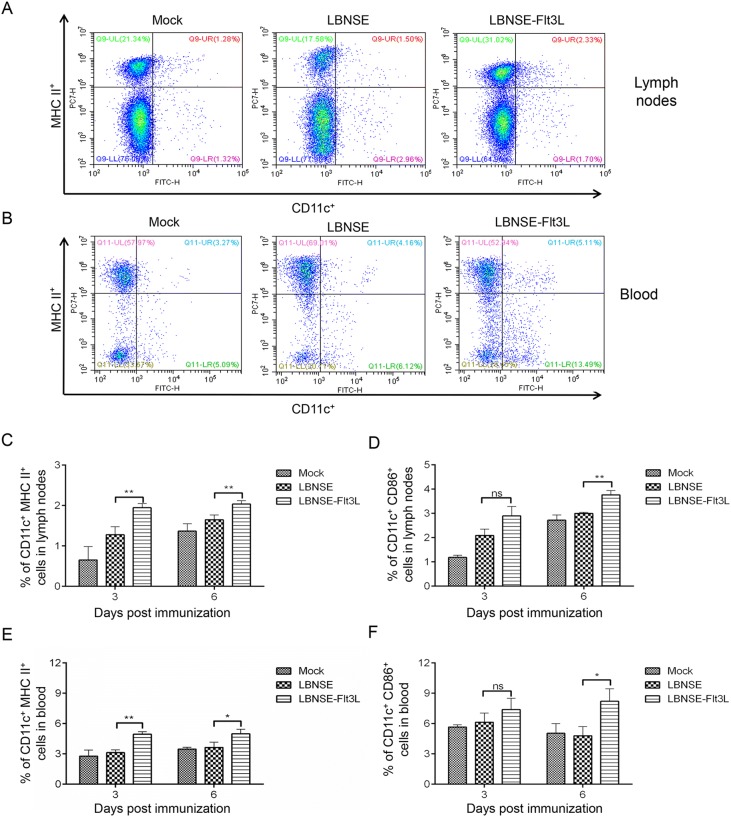
Further investigation was performed to find whether LBNSE-Flt3L could enhance the maturation of DC in vivo. Groups of mice were immunized with different rRABVs, and samples (inguinal lymph nodes and blood) were collected at 3 and 6 dpi. The representative flow cytometric plots of CD11c+ MHC II + DC in inguinal lymph nodes and blood samples were shown in Fig. 3A and 3B, respectively. For the inguinal lymph nodes (Fig. 3C and 3D), significantly more CD11c+ MHC II+ cells were detected in samples from LBNSE-Flt3L immunized mice than those from LBNSE immunized mice both at 3 and 6 dpi, while significantly more CD11c+CD86+ cells were observed at 6 dpi in LBNSE-Flt3L immunized mice than those immunized with LBNSE. For the peripheral blood (Fig. 3E, 3F), significantly more CD11c+ MHC II+ cells and CD11c+ CD86+ cells in LBNSE-Flt3L immunized mice were observed at 3 dpi, 6 dpi and 6 dpi than those in LBNSE immunized mice, respectively, which were consistent with the results in inguinal lymph nodes. Based on the results above, it was suggested that expressing of Flt3L could improve the activation of DC in immunized mice.
Fig. 3.
DC activation in different rRABVs immunized mice. Six-week-old female BALB/c mice were immunized with 1 × 106 FFU of rRABVs or the same volume of DMEM. The lymph nodes (LN) and blood samples were collected at 3 and 6 dpi. Single cell suspensions prepared from the lymph nodes and blood were analyzed for the activated DC (CD11c+ CD86+, or CD11c+ MHC II+) by flow cytometry. Representative cytometric plots for CD11c+MHC II+ DC in inguinal lymph nodes (A) and blood samples (B). Percentages of CD11c+ MHC II+ (C), or CD11c+CD86+ (D) activated DC in LN of immunized mice. Percentages of CD11c+ MHC II+ (E), or CD11c+CD86+ (F) activated DC in blood samples of immunized mice. Data are the means from three independent experiments (*P < 0.05; **P < 0.01). ns represents non-significant
Generation of TFH Cells and Germinal Center (GC) B Cells in Mice Immunized with Different rRABVs
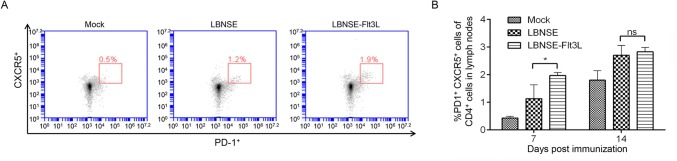
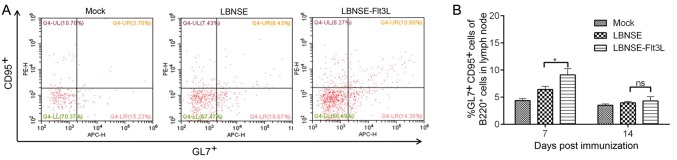
As is known that the antigen was captured and presented by APCs, such as DC, and then activated DC would interact with T cell. CD4+ naïve T cells could differentiate into different subtypes, and follicular helper T (TFH) cells, one of which play an important role in germinal center (GC) formation and generating GC B cells. To determine whether the generation of TFH and GC B cell were regulated after the activation of DC in mice immunized with different rRABVs, mice were immunized with different rRABVs or mock immunized with DMEM, and the draining lymph nodes were collected at 7 and 14 dpi. The representative flow cytometric plots of TFH (CD4+CXCR5+PD1+) in lymph nodes at 7 dpi were shown in Fig. 4A. Significantly more TFH cells were detected in lymph nodes of LBNSE-Flt3L immunized mice than those of LBNSE immunized mice at 7 dpi (Fig. 4B). For detection of GC B cells (B220+CD95+GL7+), the representative flow cytometric plots in lymph nodes of immunized mice at 7 dpi were as shown in Fig. 5A. Consistently, significantly more GC B cells were only observed at 7 dpi in lymph nodes of LBNSE-Flt3L immunized mice than those in LBNSE immunized mice (Fig. 5B). The above results revealed that the recombinant virus LBNSE-Ftl3L could enhance the generation of TFH cells and GC B cells in immunized mice.
Fig. 4.
Generation of TFH cells in mice immunized with different rRABVs. Six-week-old female BALB/c mice were infected with 1 × 106 FFU of different rRABVs or mock immunized with DMEM through i.m. route. The inguinal lymph nodes (LNs) were collected at 7 and 14 dpi. Single cell suspensions prepared from the inguinal LNs were analyzed for the generation of TFH cells (CD4+ CXCR5+ PD1+) by flow cytometry. A Representative cytometric plots for TFH cells at 7 dpi. B Percentages of TFH cells in inguinal LN from mice immunized with different rRABVs. Data are the means from three independent experiments (*P < 0.05). ns represents non-significant
Fig. 5.
Generation of Germinal Center (GC) B cells in mice immunized with different rRABVs. Six-week-old female BALB/c mice were infected with 1 × 106 FFU of different rRABVs or mock immunized with DMEM through i.m. route. The inguinal lymph nodes were collected at 7 and 14 dpi. Single cell suspensions prepared from the inguinal lymph nodes were analyzed for the generation of GC B cells (B220+ CD95+ GL7+) by flow cytometry. A Representative cytometric plots for GC B cells at 7 dpi. B Percentages of GC B cells in inguinal LN from mice immunized with different rRABVs. Data are the means from three independent experiments (*P < 0.05). ns represents non-significant
Generation of Plasma Cells in Mice Immunized with Different rRABVs
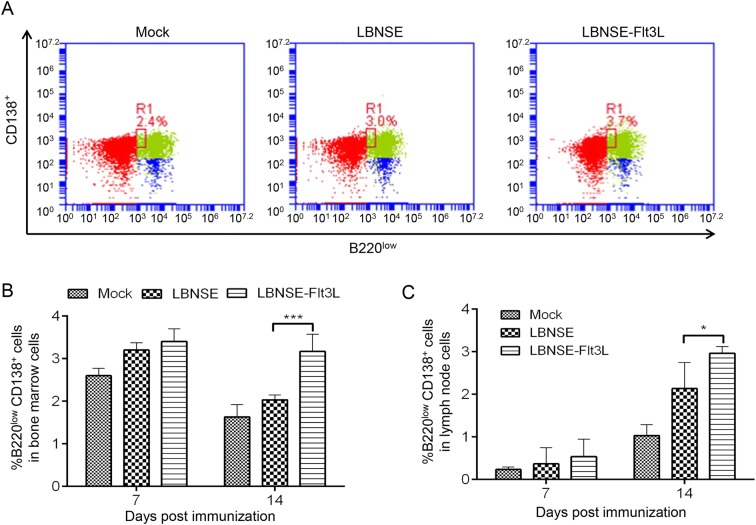
GC B cells can further differentiate into plasma cells, which are the main antibody secreting cells. Hence, to detect whether the generation of plasma cells would be affected after rRABVs immunization, mice were immunized with different rRABVs or mock immunized with DMEM, and the bone marrow and draining lymph nodes were collected at 7 and 14 dpi. The representative flow cytometric plots of plasma cells (B220lowCD138+) in bone marrow at 7 dpi were as shown in Fig. 6A. Significantly more plasma cells were detected in bone marrow and lymph nodes of mice immunized with LBNSE-Flt3L than those in LBNSE immunized mice at 14 dpi, but not 7 dpi as shown in Fig. 6B and 6C, respectively. These results suggest that expression of Flt3L could increase the plasma cells generation in immunized mice.
Fig. 6.
Generation of plasma cells in mice immunized with different rRABVs. Six-week-old female BALB/c mice were infected with 1 × 106 FFU of different rRABVs or mock immunized with DMEM through i.m. route. The bone-marrows and inguinal lymph nodes were collected at 7 and 14 dpi. Single cell suspensions prepared from the bone-marrows and inguinal lymph nodes (LNs) were analyzed for the generation of plasma cells (B220lowCD138+) by flow cytometry. A Representative cytometric plots for plasma cells in bone-marrows of immunized mice at 7 dpi. Percentages of Plasma cells in bone marrows (B) and inguinal LN (C) from mice immunized with different rRABVs. Data are the means from three independent experiments (*P < 0.05; ***P < 0.001).
VNA Induction and Protection in Different rRABVs Immunized Mice
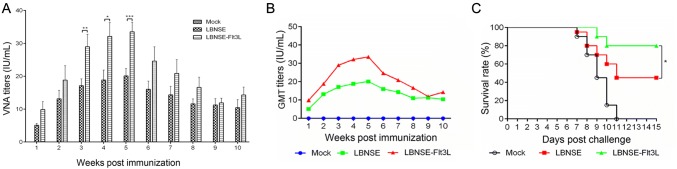
As the key indices of rabies vaccine evaluation, the ability of VNA production and survival rates after challenge were compared among the different rRABVs immunized mice. Blood samples were collected weekly from 1 week post immunization (wpi) to 10 wpi, and the sera were isolated for the determination of virus-neutralizing antibody (VNA) using the fluorescent antibody virus neutralization (FAVN) tests. As shown in Fig. 7A, significantly higher VNA titers were detected in the sera of mice vaccinated with LBNSE-Flt3L than those of mice vaccinated with LBNSE from 3 to 5 wpi; The highest geometric mean titer of VNA detected in mice immunized with LBNSE-Flt3L or LBNSE were 33.53 IU/mL (at 5 wpi) and 20.09 IU/mL (at 5 wpi), respectively (Fig. 7B). To further investigate whether the higher VNA titers were correlated to better protection, mice were challenged with 50 times of 50% lethal dose (LD50) of CVS-24 (a pathogenic RABV strain) at 3 wpi through intracranial (i.c.) route, and the mice were monitored for another 2 weeks and the survival rate for each group was recorded. It was found that only 45% of mice immunized with LBNSE were survived, while 80% of LBNSE-Flt3L immunized mice were survived from lethal challenge. Taken together, all these data indicate that LBNSE-Flt3L could promote VNA production and offer better protection than LBNSE, indicating LBNSE-Flt3L could be a promising rabies vaccine strain.
Fig. 7.
VNA production in different rRABVs immunized mice and survival rates of immunized mice after challenge. Groups of 6 weeks old female ICR mice (n = 10) were immunized with 1 × 106 FFU of LBNSE, LBNSE-Flt3L or mock immunized with the same volume of DMEM by the i.m. route. At indicated time points, blood samples were collected and sera were separated for VNA test by FAVN. The VNA titers (A) and VNA geometric mean titers (GMT) (B) were determined at different time points in mice immunized with different rRABVs. At 21 dpi, mice were challenged with 50 × LD50 of CVS-24 by i.c. route and observed twice a day for 21 days, and the survival rates curve was depicted based on the numbers of survivors (C). Data are the means from three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001).
Discussion
Rabies is still a threat to public health, especially in Asia and Africa (Okonko et al.2010; Organization 2013). Since vaccination is the most effective way to prevent rabies, developing efficacious and affordable vaccine is very meaningful for rabies control. Previous studies demonstrated that the expression of GM–CSF by recombinant RABV could enhance the immunogenicity by recruiting and/or activating more DC after vaccination in mice (Wen et al.2011; Zhou et al.2013, 2015). It was known that Flt3L, similar with GM–CSF, plays an important role in the development and expansion of DC (Kreiter et al.2011, 2016). To further characterize the role of DC activation that plays in the immunogenicity of rabies vaccine, an rRABV expressing Flt3L was constructed and characterized in this study.
For developing a good attenuated rabies vaccine, the vaccine virus titer and pathogenicity are two important characteristics. Therefore, the replication and pathogenicity of rRABV expressing Flt3L (LBNSE-Flt3L) were assessed by depicting the viral growth curves in BSR cells and body-weight changes in infected mice, respectively, in our study. Previous study demonstrated that the expression of Flt3L by recombinant vaccinia virus (rVACV) highly decreased the growth of rVACV in macrophage cell line in vitro as well as its multiplication in vivo when inoculated in mice (Zurkova et al.2010); nevertheless, similar replication characteristics were observed between LBNSE-Flt3L and parent virus LBNSE in BSR cells in our study. The possible reason for this is that the influence of Flt3L on viral replication is virus specific and cell type specific as well, since the study also found that the Flt3L did not affect rVACV multiplication in HeLa cells (Zurkova et al.2010). For pathogenicity, there was a slight increase on body-weight changes of mice infected with LBNSE-Flt3L when compared with those infected with parent virus LBNSE, although the change was statistically not significant. Hence, the expression of Flt3L did not affect the viral replication in its culture cells and slightly decrease the pathogenicity, indicating that LBNSE-Flt3L could be further developed as an attenuated rabies vaccine.
The effects of Flt3L on anti-tumor and autoimmunity have been widely studied previously due to its ability to enhance the CD8+ T cell response and facilitate formation of regulatory T (Treg) cells, respectively, and increase the proliferation of DC as well (Svensson et al.2013; Gao et al.2018; Miao et al.2018). In addition, Flt3L was also widely used as an adjuvant of DNA vaccines to enhance the immunogenicity, especially the cellular immunity (Zhou et al.2010; Mwangi et al.2011; Xu et al.2016; Gao et al.2018). In our study, we further assessed the potential of Flt3L as an adjuvant to enhance the humoral immune responses to live rabies vaccine, and it was found that the expression of Flt3L by rRABV could enhance the VNA production and improve the survival rate of mice after i.m. immunization, which is comparable to the previously characterized vaccine strain that expressing mouse GM–CSF (Wen et al.2011; Zhou et al.2013). As is known, VNA is one of the most critical factors correlated to protection from rabies infection (Hooper et al.1998). Although T cells play a very important role in controlling virus infection by the host, mice lacking CD8+ T cells showed no significant differences in the development of clinical signs after RABV strain CVS-F3 infection by comparison with intact counterparts having the same genetic background (Hooper et al.1998). Furthermore, for rabies, control of the infection by T cells is inefficient and is specifically inactivated by the virus (Lafon 2008). In another word, the humoral immune response rather than cellular immune response is crucial for the rabies control. Therefore, in this study, only TFH cells (a subset of CD4+ helper T cells) that could regulate the development of antigen-specific B cell immunity were investigated (Nurieva et al.2008; Morita et al.2011).
Additionally, Flt3L has also been investigated in other immunization routes beyond parenteral immunization (Pabst et al.2003; Kodama et al.2010). Due to the main source of human rabies in developing countries is dogs, especially stray dogs (Sudarshan et al.2007; Song et al.2014), it would be very meaningful to develop oral rabies vaccine. It was demonstrated that nasal application of Flt3L could induce an increase in the number of dendritic cells in nasal-associated lymphoid tissue and enhance the production of antigen-specific antibody to accelerate the pathogen clearance (Kodama et al.2010). Since the potential of Flt3L as an effective mucosal adjuvant, the rRABV LBNSE-Flt3L would be investigated in oral or intranasal immunization in our future study.
In summary, the rRABV LBNSE-Flt3L is capable of activating DC in vitro and in vivo, and it could enhance the generation of TFH, GC B and plasma cells to produce significantly higher VNA after intramuscular immunization, indicating that it has the potential to be developed as an efficient rabies vaccine. Meanwhile, the results of this study could further illustrate the importance of DC activation in enhancing the immunogenicity of rabies vaccine.
Acknowledgements
This study was partially supported by the National Program on Key Research Project of China (2016YFD0500400 and 2017YFD0501701), the National Natural Science Foundation of China (31872494, 31402176, 31372419, and 31522057), the Fundamental Research Funds for the Central Universities (No. 2662016QD036 to MZ), the Ministry of Science and Technology of China (863 program, No. 2011AA10A212), and the Ministry of Agriculture of China (Special Fund for Agro-scientific Research in the Public Interest, No. 201303042 to ZFF).
Author Contributions
Experimental design was conducted by MZ and LZ. Experiments were finished by YZ, JY, and ML. Data analysis was conducted by MZ, LZ, ZFF, and MC. The manuscript was written by YZ, and revised by MZ and LZ.
Compliance with Ethics Standards
Animal and Human Rights Statement
The animal experiments were carried out in strict accordance with the protocols approved by The Scientific Ethics Committee of Huazhong Agricultural University (permit number: HZAUMO-2015-029). All institutional and national guidelines for the care and use of laboratory animals were followed.
Footnotes
Yachun Zhang and Jie Yang have contributed equally to this work.
Contributor Information
Ling Zhao, Email: lingzhao@mail.hzau.edu.cn.
Ming Zhou, Email: mikchail@163.com.
References
- Abelaridder B. Rabies: 100 per cent fatal, 100 per cent preventable. Vet Rec. 2015;177:148–149. doi: 10.1136/vr.h4196. [DOI] [PubMed] [Google Scholar]
- Acharya AS, Kaur R, Lakra K. Rabies epidemiology and control in India: a review. J Commun Dis. 2012;44:59–69. [PubMed] [Google Scholar]
- Adedeji AO, Okonko IO, Eyarefe OD, Adedeji OB, Babalola ET, Ojezele MO, Nwanze JC, Amusan TA. An overview of rabies—history, epidemiology, control and possible elimination. Afr J Microbiol Res. 2010;4:2327–2338. [Google Scholar]
- Adolfsson J, Borge JO, Bryder D, TheilgaardMönch K, ÅstrandGrundström I. Upregulation of Flt3 expression within the bone marrow Lin − Sca1 + c-kit + stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/S1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- Alizadeh L, Dana MA, Dowom PB, Ghaemi A. Immunology of rabies virus in the central nervous system. Osong Public Health Res Perspect. 2015;3:113–120. [Google Scholar]
- Anandasabapathy N, Feder R, Mollah S, Tse SW, Longhi MP, Mehandru S, Matos I, Cheong C, Ruane D, Brane L. Classical Flt3L-dependent dendritic cells control immunity to protein vaccine. J Exp Med. 2014;211:1875–1891. doi: 10.1084/jem.20131397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JA, Barraviera B, Calvi SA, Carvalho NR, Peraçoli MTS. Antibody and cytokine serum levels in patients subjected to anti-rabies prophylaxis with serum-vaccination. J Venom Anim Toxins Incl Trop Dis. 2006;12:435–455. doi: 10.1590/S1678-91992006000300008. [DOI] [Google Scholar]
- Chen T, Zhang Y, Wang Z, Yang J, Li M, Wang K, Cui M, Fu ZF, Zhao L, Zhou M. Recombinant rabies virus expressing IL-15 enhances immunogenicity through promoting the activation of dendritic cells in mice. Virol Sin. 2017;32:317–327. doi: 10.1007/s12250-017-4036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology. 1990;175:485–499. doi: 10.1016/0042-6822(90)90433-R. [DOI] [PubMed] [Google Scholar]
- Dodet B. Report of the sixth AREB meeting, Manila, The Philippines, 10–12 November 2009. Vaccine. 2010;28:3265–3268. doi: 10.1016/j.vaccine.2010.02.093. [DOI] [PubMed] [Google Scholar]
- Dupont CD, Pritchard GH, Hidano S, Christian DA, Wagage S, Muallem G, Wojno EDT, Hunter CA. Flt3L is essential for survival and protective immune responses during toxoplasmosis. J Immunol. 2015;195:4369–4377. doi: 10.4049/jimmunol.1500690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FS, Zhan YT, Wang XD, Zhang C. Enhancement of anti-tumor effect of plasmid DNA-carrying MUC1 by the adjuvanticity of FLT3L in mouse model. Immunopharmacol Immunotoxicol. 2018;40:1–5. doi: 10.1080/08923973.2018.1498099. [DOI] [PubMed] [Google Scholar]
- Garg R, Shrivastava P, Van Drunen Littel-van den Hurk S. The role of dendritic cells in innate and adaptive immunity to respiratory syncytial virus, and implications for vaccine development. Expert Rev Vaccines. 2012;11:1441–1457. doi: 10.1586/erv.12.117. [DOI] [PubMed] [Google Scholar]
- Ge Y, Waldemer RJ, Nalluri R, Nuzzi PD, Chen J. Flt3L is a novel regulator of skeletal myogenesis. J Cell Sci. 2013;126:3370–3379. doi: 10.1242/jcs.123950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E. DC-based cancer vaccines. J Clin Investig. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998;72:3711–3719. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Tang Q, Tang J, Fooks AR. Rabies in China: an update. Vector Borne Zoonotic Dis. 2009;9:1–12. doi: 10.1089/vbz.2008.0046. [DOI] [PubMed] [Google Scholar]
- Kodama S, Hirano T, Noda K, Abe N, Suzuki M. A single nasal dose of fms-like tyrosine kinase receptor-3 ligand, but not peritoneal application, enhances nontypeable Haemophilus influenzae-specific long-term mucosal immune responses in the nasopharynx. Vaccine. 2010;28:2510–2516. doi: 10.1016/j.vaccine.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Kreiter S, Diken M, Selmi A, Diekmann J, Attig S, Husemann Y, Koslowski M, Huber C, Tureci O, Sahin U. FLT3 ligand enhances the cancer therapeutic potency of naked RNA vaccines. Cancer Res. 2011;71:6132–6142. doi: 10.1158/0008-5472.CAN-11-0291. [DOI] [PubMed] [Google Scholar]
- Kreiter S, Diken M, Selmi A, Petschenka J, Tureci O, Sahin U. FLT3 ligand as a molecular adjuvant for naked RNA vaccines. Methods Mol Biol. 2016;1428:163–175. doi: 10.1007/978-1-4939-3625-0_11. [DOI] [PubMed] [Google Scholar]
- Lafon M. Immune evasion, a critical strategy for rabies virus. Dev Biol (Basel) 2008;131:413–419. [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- Mathers AR, Larregina AT. Professional antigen-presenting cells of the skin. Immunol Res. 2006;36:127–136. doi: 10.1385/IR:36:1:127. [DOI] [PubMed] [Google Scholar]
- Miao X, Chen Y, Hao K, Zheng M, Chen B, Li K, Wang Y, Zhang W, Zhang Y, Mou X, Jiang S, Wang Z. CD103(+) cell growth factor Flt3L enhances the efficacy of immune checkpoint blockades in murine glioblastoma model. Oncol Res. 2018;26:173–182. doi: 10.3727/096504017X14841698396865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. Human blood CXCR24(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FA, Bauer SP. Early street rabies virus infection in striated muscle and later progression to the central nervous system. Intervirology. 1974;3:256–268. doi: 10.1159/000149762. [DOI] [PubMed] [Google Scholar]
- Mwangi DM, Honda Y, Graham SP, Pelle R, Taracha EL, Gachanja J, Nyanjui JK, Bray J, Palmer GH, Brown WC, Mwangi W. Treatment of cattle with DNA-encoded Flt3L and GM–CSF prior to immunization with Theileria parva candidate vaccine antigens induces CD4 and CD8 T cell IFN-gamma responses but not CTL responses. Vet Immunol Immunopathol. 2011;140:244–251. doi: 10.1016/j.vetimm.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonko IO, Adedeji OB, Babalola ET, Fajobi EA, Fowotade A, Adewale OG. Why is there still rabies in the world?—An emerging microbial and global health threat. Glob Vet. 2010;4:34–50. [Google Scholar]
- Pabst R, Luhrmann A, Steinmetz I, Tschernig T. A single intratracheal dose of the growth factor Fms-like tyrosine kinase receptor-3 ligand induces a rapid differential increase of dendritic cells and lymphocyte subsets in lung tissue and bronchoalveolar lavage, resulting in an increased local antibody production. J Immunol. 2003;171:325–330. doi: 10.4049/jimmunol.171.1.325. [DOI] [PubMed] [Google Scholar]
- Rasalingam P, Rossiter JP, Mebatsion T, Jackson AC. Comparative pathogenesis of the SAD-L16 strain of rabies virus and a mutant modifying the dynein light chain binding site of the rabies virus phosphoprotein in young mice. Virus Res. 2005;111:55–60. doi: 10.1016/j.virusres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Schnell MJ, Mebatsion T, Conzelmann KK. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotch M, Odofin L, Rabinowitz P. Linkages between animal and human health sentinel data. BMC Vet Res. 2009;5:15–24. doi: 10.1186/1746-6148-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Tang Q, Rayner S, Tao XY, Li H, Guo ZY, Shen XX, Jiao WT, Fang W, Wang J, Liang GD. Human rabies surveillance and control in China, 2005–2012. BMC Infect Dis. 2014;14:212–221. doi: 10.1186/1471-2334-14-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NS, Ashwath Narayana DH, Abdul Rahman S, Meslin F, Lobo D, Ravikumar K, Gangaboraiah Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Int J Infect Dis. 2007;11:29–35. doi: 10.1016/j.ijid.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Svensson MN, Andersson SE, Erlandsson MC, Jonsson IM, Ekwall AK, Andersson KM, Nilsson A, Bian L, Brisslert M, Bokarewa MI. Fms-like tyrosine kinase 3 ligand controls formation of regulatory T cells in autoimmune arthritis. PLoS ONE. 2013;8:e54884. doi: 10.1371/journal.pone.0054884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Luo Z, Zhou M, Li M, Yu L, Wang C, Yuan J, Li F, Tian B, Sui B. Critical role of K1685 and K1829 in the large protein of rabies virus in viral pathogenicity and immune evasion. J Virol. 2015;90:232–244. doi: 10.1128/JVI.02050-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskow C, Liu K, Darrassejèze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Wang H, Wu H, Yang F, Tripp RA, Hogan RJ, Fu ZF. Rabies virus expressing dendritic cell-activating molecules enhances the innate and adaptive immune response to vaccination. J Virol. 2011;85:1634–1644. doi: 10.1128/JVI.01552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2013) WHO expert consultation on rabies. Second report. World Health Organ Tech Rep Ser 982:1 [PubMed]
- Wu X, Smith TG, Franka R, Wang M, Carson WC, Rupprecht CE. The feasibility of rabies virus-vectored immunocontraception in a mouse model. Trials Vaccinol. 2014;3:11–18. doi: 10.1016/j.trivac.2013.11.003. [DOI] [Google Scholar]
- Wunner WH, Briggs DJ. Rabies in the 21 century. PLoS Negl Trop Dis. 2010;4:e591. doi: 10.1371/journal.pntd.0000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Zhu YF, Wang HC, Gong ZW, Yu YZ. Enhanced efficacy of DNA vaccination against botulinum neurotoxin serotype A by co-administration of plasmids encoding DC-stimulating Flt3L and MIP-3alpha cytokines. Biologicals. 2016;44:441–447. doi: 10.1016/j.biologicals.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Zhao L, Toriumi H, Kuang Y, Chen HC, Fu ZF. The roles of chemokines in rabies virus infection: overexpression may not always be beneficial. J Virol. 2009;83:11808–11818. doi: 10.1128/JVI.01346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wang F, Yang F, Wang Y, Zhang X, Sun S. Augmented humoral and cellular immune response of hepatitis B virus DNA vaccine by micro-needle vaccination using Flt3L as an adjuvant. Vaccine. 2010;28:1357–1362. doi: 10.1016/j.vaccine.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Zhou M, Zhang G, Ren G, Gnanadurai CW, Li Z, Chai Q, Yang Y, Leyson CM, Wu W, Cui M, Fu ZF. Recombinant rabies viruses expressing GM–CSF or flagellin are effective vaccines for both intramuscular and oral immunizations. PLoS ONE. 2013;8:e63384. doi: 10.1371/journal.pone.0063384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Wang L, Zhou S, Wang Z, Ruan J, Tang L, Jia Z, Cui M, Zhao L, Fu ZF. Recombinant rabies virus expressing dog GM–CSF is an efficacious oral rabies vaccine for dogs. Oncotarget. 2015;6:38504–38516. doi: 10.18632/oncotarget.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkova K, Hainz P, Krystofova J, Kutinova L, Sanda M, Nemeckova S. Attenuation of vaccinia virus by the expression of human Flt3 ligand. Virol J. 2010;7:109–124. doi: 10.1186/1743-422X-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]