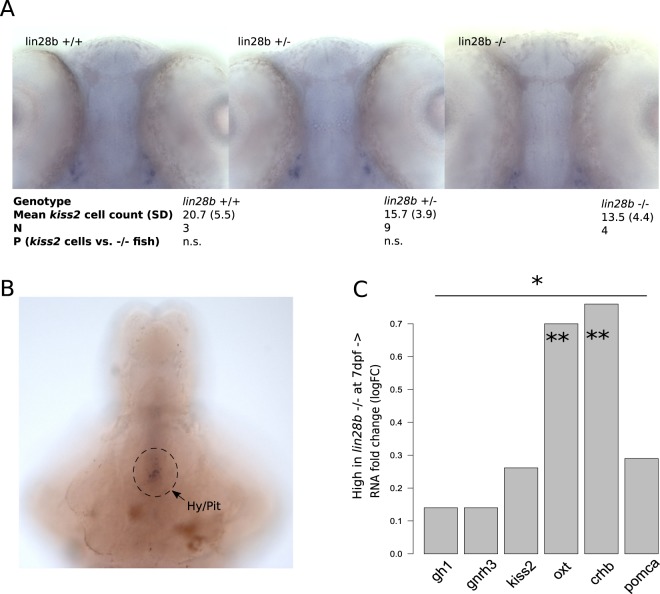
Figure 2.
Evaluation of the lin28b KO effects on the HP axis in zebrafish larvae. (A) Representative images of kiss2 expression at 3 dpf in the control (+/+), the heterozygous (+/−), and the lin28b KO (−/−) zebrafish. Though this varied between individual samples, the staining location and intensity was comparable between the groups. Despite a trend towards less kiss2 expressing cells in the KO vs. control fish, we did not detect significant differences in the neuron numbers between the samples (Welch Two Sample t-test). The analysis was complicated by the fact that the +/+ group consists of WT fish from the exon3 crosses, whereas 3 out of 4 −/− fish were obtained from exon1 crosses. As the gene expression data from other experiments indicated that kiss2 transcripts are not significantly up- or downregulated in the KO fish, we concluded that the lin28b KO does not prevent normal development of the kiss2 neurons (e.g. C, Supplementary data 2, and Fig. 3). (B) Image of 7 dpf +/+ zebrafish brain showing weak staining by lin28b anti-sense mRNA in the hypothalamic-pituitary region. (C) HP axis RNA expression was slightly upregulated in the KO fish at 7 dpf. The analysis was restricted to hormonal genes that were expressed in high enough quantities to pass filtering in the RNA-seq experiment. Oxytocin (oxt) and corticotrophin-releasing-hormone beta (chrb) appeared upregulated in the KO samples (**p < 0.005, and overall the expression of the studied HP axis genes appeared upregulated in the lin28b KO zebrafish based on the RNAseq data from 7 dpf (*p < 0.05) (Welch two-sample t-test).