Table 1.
| SPARTAN Jannsen |
PROSPER Pfizer/Astellas |
ARAMIS ORION/Bayer HealthCare |
||||
|---|---|---|---|---|---|---|
| Apalutamide, Erleada | Enzalutamide, Extandi® | Darolutamide | ||||
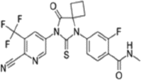
|
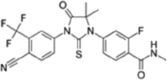
|
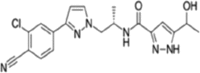
|
||||
| Group | A | P | E | P | D | P |
| Patients, n | 806 | 421 | 933 | 468 | 955 | 554 |
| M-Fs, m | 40 | 18 | 37 | 15 | 40 | 18 |
| SAE’s, % | 25 | 20 | 31 | 23 | 25 | 20 |
| Discontinuation, % | 11 | 7 | 10 | 6 | 9 | 9 |
All subjects were on continuous conventional androgen deprivation therapy. Values are rounded
A apalutamide, E enzalutamide, D, darolutamide, P placebo, M-FS metastasis-free survival, months, SAE’s serious adverse events
