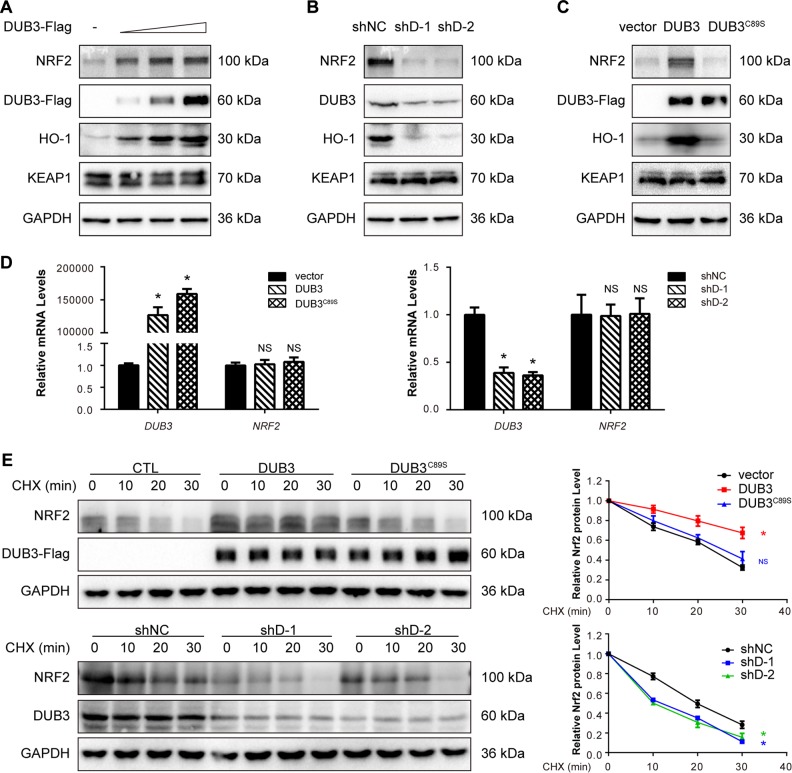
Fig. 2.
DUB3 stabilizes NRF2 protein. a Overexpression of DUB3 increases the endogenous NRF2 protein level in a dose-dependent manner. HCT116 cells were transfected with the indicated amounts of DUB3-Flag plasmids. Forty-eight hours after transfection, the cell lysates were analyzed by western blot with the indicated antibodies. The endogenous NRF2 protein expression was normalized to GAPDH. b DUB3 deficiency decreases the endogenous expression of NRF2. HCT116 cells were transfected with the indicated shRNAs. Forty-eight hours after transfection, the cell lysates were analyzed by western blot with the indicated antibodies. c The DUB3 catalytic mutant (DUB3C89S) lacks the ability to increase NRF2 protein levels. HCT116 cells were transfected with the indicated plasmids. Forty-eight hours after transfection, the cell lysates were analyzed by western blot with the indicated antibodies. d DUB3 has no effect on the NRF2 mRNA level. HCT116 cells were transfected with DUB3 expression or shRNA vectors. mRNA was isolated, and qPCR was performed 48 h after transfection. The NRF2 mRNA level was normalized to GAPDH, and the controls were set equal to 1. Data were analyzed employing one-way ANOVA and presented as the mean ± SD (N = 3/group). e The NRF2 half-life is affected by DUB3. Left: HCT116 cells were transfected with the indicated plasmids and treated with 50 µg/mL CHX for the indicated times at 12 h posttransfection. Cells were harvested for immunoblotting with the indicated antibodies. Right: quantification of the NRF2 protein level normalized to GAPDH. Data were analyzed employing one-way ANOVA and presented as the mean ± SD (N = 3/group)