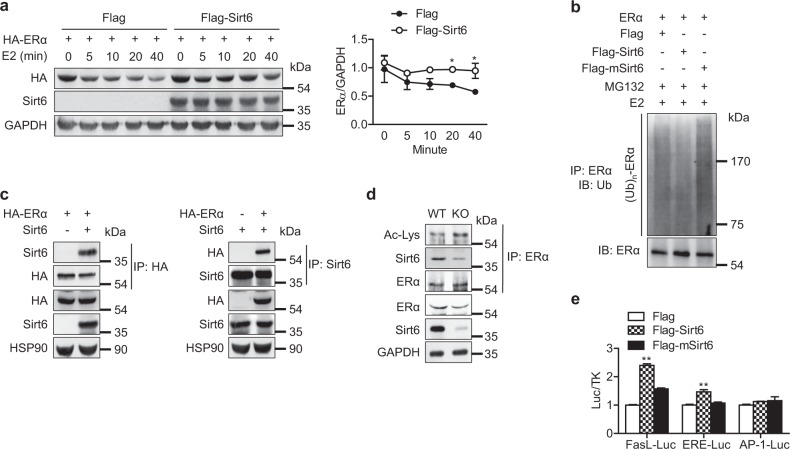
Fig. 7.
Blockade of ubiquitin-proteasomal degradation of ERα by Sirt6. a HEK293T cells transfected with Era were treated with estrogen (E2, 10 nM) for the indicated time, and the relative protein levels of ERα were compared (n = 3). b HEK293T cells were transfected with Era and Sirt6 or deacetylase-lacking mutant Sirt6 (mSirt6) as indicated. After 24 h, the cells were treated with E2 (10 nM) for 40 min in the presence of MG132 (2 μM), and total cell lysates were immunoprecipitated with anti-ERα antibodies and immunoblotted with anti-ubiquitin antibodies. c After transfection in HEK293T cells as indicated, a co-immunoprecipitation assay to determine the physical interaction between Sirt6 and ERα was performed. d CD11b+ BMCs from WT or mS6KO mice were treated with M-CSF and RANKL for 3 days, and whole cell lysates were immunoprecipitated with anti-ERα antibody and subjected to western blot to detect ERα acetylation. e HEK293T cells were transfected with WT or mutant Sirt6, and the FasL, ERE and AP1 luciferase activity in the cell lysates was measured (n = 4). The values are mean ± SEM. *p < 0.05 and **p < 0.01 versus Flag