Table 1. Selected optimization studies a .
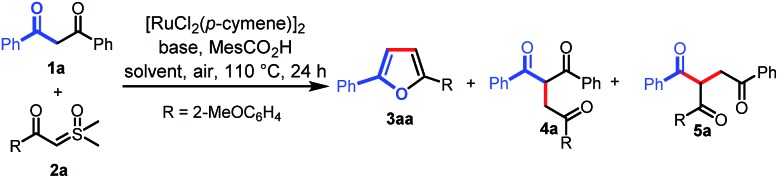
| ||||
| Entry | Solvent | Base | Yield
b
(%) |
|
| 3aa | 4a + 5a (4a : 5a) | |||
| 1 | HFIP | Na3PO4 | 23 | 45 (1 : 6) |
| 2 | iPrOH | Na3PO4 | 26 | 8 (1 : 3) |
| 3 | DMF | Na3PO4 | 30 | 20 (1 : 3) |
| 4 | CH3CN | Na3PO4 | 25 | 14 (1 : 2) |
| 5 | DCE | Na3PO4 | 15 | 24 (1 : 5) |
| 6 | Toluene | Na3PO4 | 35 | Trace |
| 7 | Toluene | Na2CO3 | 30 | Trace |
| 8 | Toluene | K2CO3 | 20 | Trace |
| 9 | Toluene | Cs2CO3 | 25 | Trace |
| 10 | Toluene | NaHCO3 | 24 | 0 |
| 11 | Toluene | KH2PO4 | 52 | 0 |
| 12 | Toluene | tBuOLi | 72 | Trace |
| 13 | Toluene | — | 10 | 0 |
| 14 c | Toluene | tBuOLi | Trace | 0 |
| 15 c | Toluene | MesCO2Li | 71 | Trace |
| 16 c , d | Toluene | MesCO2Li | 78 | Trace |
| 17 c , e | Toluene | MesCO2Li | 40 | Trace |
| 18 c , d , f | Toluene | MesCO2Li | 85(82) g | Trace |
| 19 c , d , h | Toluene | MesCO2Li | 66 | Trace |
| 20 c , d , i | Toluene | MesCO2Li | 0 | 0 |
aReaction conditions: except where otherwise noted, all of the reactions were performed with 1a (0.1 mmol), 2a (0.2 mmol), base (0.15 mmol), MesCO2H (0.15 mmol), and [RuCl2(p-cymene)]2 (5 mol%) in a solvent (1 mL) at 110 °C in air for 24 h.
bThe yields were determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard.
cWithout MesCO2H.
dReaction was carried out at 120 °C.
eReaction was carried out at 130 °C.
f2 mL of toluene was used.
gIsolated yield.
h3 mL of toluene was used.
iWithout [RuCl2(p-cymene)]2. HFIP = 1,1,1,3,3,3-hexafluoro-2-propanol. DMF = N,N-dimethylformamide. DCE = 1,2-dichloroethane. MesCO2H = 2,4,6-trimethylbenzoic acid.
