Weight history, along with current weight, is useful for pediatricians tasked with assessing illness severity in today’s diverse population of patients with restrictive EDs.
Abstract
BACKGROUND:
Lower weight has historically been equated with more severe illness in anorexia nervosa (AN). Reliance on admission weight to guide clinical concern is challenged by the rise in patients with atypical anorexia nervosa (AAN) requiring hospitalization at normal weight.
METHODS:
We examined weight history and illness severity in 12- to 24-year-olds with AN (n = 66) and AAN (n = 50) in a randomized clinical trial, the Study of Refeeding to Optimize Inpatient Gains (www.clinicaltrials.gov; NCT02488109). Amount of weight loss was the difference between the highest historical percentage median BMI and admission; rate was the amount divided by duration (months). Unpaired t tests compared AAN and AN; multiple variable regressions examined associations between weight history variables and markers of illness severity at admission. Stepwise regression examined the explanatory value of weight and menstrual history on selected markers.
RESULTS:
Participants were 16.5 ± 2.6 years old, and 91% were of female sex. Groups did not differ by weight history or admission heart rate (HR). Eating Disorder Examination Questionnaire global scores were higher in AAN (mean 3.80 [SD 1.66] vs mean 3.00 [SD 1.66]; P = .02). Independent of admission weight, lower HR (β = −0.492 [confidence interval (CI) −0.883 to −0.100]; P = .01) was associated with faster loss; lower serum phosphorus was associated with a greater amount (β = −0.005 [CI −0.010 to 0.000]; P = .04) and longer duration (β = −0.011 [CI −0.017 to 0.005]; P = .001). Weight and menstrual history explained 28% of the variance in HR and 36% of the variance in serum phosphorus.
CONCLUSIONS:
Weight history was independently associated with markers of malnutrition in inpatients with restrictive eating disorders across a range of body weights and should be considered when assessing illness severity on hospital admission.
What’s Known on This Subject:
Lower weight was traditionally equated with more severe illness in anorexia nervosa. The rapid rise in patients with atypical anorexia nervosa who require hospitalization at normal weight has challenged reliance on current weight to guide clinical concern.
What This Study Adds:
Patients with large, rapid, or long-duration of weight loss were more severely ill regardless of their current weight. Weight history can help guide clinical concern for pediatricians tasked with assessing restrictive eating disorders in today’s diverse population of adolescents.
Lower weight was traditionally equated with more severe illness in anorexia nervosa (AN). A higher index of concern was supported by extensive evidence associating low weight with worse bradycardia and hypotension,1,2 higher risk for refeeding hypophosphatemia,3–6 lower bone mineral density,1 hematologic abnormalities,2 and elevated liver enzymes.7 In addition to more severe medical instability and malnutrition, low weight was required for a psychiatric diagnosis of AN before 2013.8 Accurate assessment of illness severity is critical on hospital admission to inform treatment decisions, such as approaches to refeeding and frequency of electrolyte monitoring.9–12
Recognition of atypical anorexia nervosa (AAN)8 has challenged reliance on current weight to assess illness severity. Patients with AAN have eating disorder (ED) psychopathology and significant weight loss but are not underweight relative to national norms. The number of patients with AAN requiring medical hospitalization has risen sharply to comprise approximately one-third of hospital inpatient ED programs.13–15 In the only available study to comprehensively compare illness severity in adolescents diagnosed with AAN versus AN, Sawyer et al15 reported a greater magnitude of weight loss over a longer period, equal bradycardia and orthostasis, and worse ED psychopathology. Studies including normal-weight patients with ED before the recognition of AAN in 2013 have reported similar findings.13,16,17
Weight suppression (WS), the difference between premorbid and presentation weight, has emerged from the adult AN literature as a marker of illness severity18 and predictor of treatment outcomes.19 This is reflected in updated recommendations, which now include percentage of weight loss and time frame in the assessment of malnutrition.20,21 Studies of normal-weight patients with ED before 2013 documented associations between greater WS and worse bradycardia,16,17 lower plasma phosphate,17 and persistent amenorrhea.22 Recently, Whitelaw et al14 examined WS in AAN and identified total and recent weight loss as predictors of bradycardia independent of presentation weight.14 Although these findings support WS as an indicator of medical instability in AAN, the association between WS and overall illness severity requires examination. Other weight history variables, including rate and duration of loss, have not been investigated in AAN. A faster rate of weight loss has been examined in normal-weight adolescents with ED and associated with worse bradycardia.17 Longer duration has been examined extensively in AN and associated with lower heart rate (HR), systolic blood pressure (BP), and bone mineral density.1,2 Our objective in this study was to examine the independent and collective relationships between comprehensive weight history (amount, rate, and duration of loss) and medical, nutritional, and psychological status. We hypothesized that participants with more dramatic weight losses would be more severely ill on hospitalization regardless of their admission weight.
Methods
Participants and Data Collection
Participants
Inclusion criteria were age 12 to 24 years, being admitted to the hospital per Society for Adolescent Health and Medicine criteria21 with AN or AAN, and being >60% of the median BMI (mBMI), defined as the 50th percentile BMI for age and sex.23 Because the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition does not identify a weight threshold for AAN, mBMI >85% was applied. Patients were excluded if they were admitted to the hospital in the previous 6 months, were currently pregnant or diagnosed with bulimia nervosa,8 or had chronic disease, suicidality or psychosis. Figure 1 shows that 301 patients met inclusion criteria from February 2016 to March 2019, 120 enrolled in this multicenter randomized clinical trial, the Study of Refeeding to Optimize Inpatient Gains (www.clinicaltrials.gov; NCT02488109), and 181 declined, most commonly because they preferred nonstudy treatment (32%) or were uninterested in research (21%). Four were ineligible postenrollment, leaving a final count of 116. Baseline data are reported here, collected within 24 hours of admission before the intervention. Study sites included clinical centers at the University of California, San Francisco Benioff Children’s Hospital; Lucile Packard Children’s Hospital at Stanford; and the Data Coordinating Center. The institutional review boards at each site approved the study.
FIGURE 1.
Flow of participants into the Study of Refeeding to Optimize Inpatient Gains.
Demographic Data
Race, ethnicity, and socioeconomic information were self-reported by participants and parents on admission. Socioeconomic status (SES) scores were derived from the Hollingshead occupational index for mother or primary parent (A.B. Hollingshead, PhD, unpublished observations, 1975), which is validated as a single-factor approach to derive SES.24
Weight and Menstrual History
Highest historical weight, height, and last menstrual period and dates of those measures were self-reported by proctored questionnaire and corroborated with parent reports, growth records, and physician history. Historical BMI was calculated by using historical weight and height; percentage mBMI was BMI divided by mBMI and multiplied by 100.
ED Psychopathology
The Eating Disorder Examination Questionnaire (EDE-Q)25 was self-administered; scores range from 1 to 6, with higher scores indicating greater severity.
Anthropometric, Vital Sign, and Laboratory Data
Weight was measured on an electronic mobile stand-on scale in the morning, postvoiding and in a gown only. Height was measured once on a stadiometer. Oral temperature was taken once per day orally, automated BP and HR measures were taken every 4 to 8 hours, night HR was measured with continuous cardiac monitoring, and postural changes of HR and BP were assessed at least twice per day, with supine measurements (after 5 minutes’ rest) followed by standing measurements (after 2 minutes). When multiple measures were taken within 24 hours, the most deviant value (eg, lowest BP) was recorded. Blood was drawn once by venipuncture between 5:00 am and 7:00 am; samples were analyzed on-site.
Statistical Methods
AN and AAN were compared with unpaired t test results for continuous variables or Fisher’s exact tests for categorical variables (eg, race and/or ethnicity). Associations between 3 weight history variables (independent variables) and indicators of illness severity on admission (dependent variables) were examined with multiple variable regression models. (1) Amount of loss was the change in the percentage of mBMI from the highest historical percentage of mBMI to the admission percentage of mBMI. (2) Rate of loss was the amount of loss divided by the duration of weight loss. (3) Duration of loss was the time (in months) between the highest historical weight and the date of admission. Models were adjusted for admission percentage of mBMI. Multivariable linear regression, followed by stepwise backward selection, was used to examine the contribution of comprehensive weight history on 3 illness severity outcomes used clinically as indicators of medical instability (HR), nutritional risk (serum phosphorus), and psychological symptoms (EDE-Q global score). Weight history models began with 10 independent variables: all 8 weight history variables are shown in Table 1 along with age and admission percentage of mBMI in the full study population. Amenorrhea and weight history models began with 11 independent variables: the same 10 variables in the weight history models plus duration of amenorrhea. These analyses were restricted to the 50 postmenarcheal girls with ≥3 months since their last menstrual period and who were not taking hormonal contraception. Collinear weight variables were included to explain as much of the variance in the outcome as possible. Although collinearity affects individual coefficients and P values, it does not influence the precision of the prediction or the goodness-of-fit statistic.26 A significance level of 0.20 was set for variable removal to avoid excluding potentially important variables.
TABLE 1.
Characteristics of Study Participants on Admission, Including Demographics, Wt and Menstrual History, ED Psychopathology, and Vital Sign and Laboratory Values
| AAN (n = 50) | AN (n = 66) | P | |
|---|---|---|---|
| Demographics | |||
| Age, y, mean ± SD | 16.3 ± 2.6 | 16.7 ± 2.5 | .36 |
| Female sex, % (n) | 88 (44) | 92 (61) | .53 |
| Race and/or ethnicity, % (n) | .49 | ||
| Non-Hispanic white | 50 (25) | 67 (44) | |
| Asian American | 12 (6) | 12 (8) | |
| Hispanic or Latino | 26 (13) | 17 (11) | |
| Other or >1 race and/or ethnicity | 12 (6) | 5 (3) | |
| Family history of ED, % | 32 (16) | 30 (20) | .55 |
| Socioeconomic score, mean ± SD | 4.83 ± 3.8 | 5.27 ± 3.0 | .51 |
| Wt and menstrual history | |||
| Highest historical BMI, mean ± SD | 25.2 ± 5.6 | 20.7 ± 3.0 | <.001 |
| Highest historical % mBMI, mean ± SD | 129.2 ± 28.8 | 104.3 ± 15.9 | <.001 |
| Wt loss, kg, mean ± SD | 14.5 ± 12.0 | 12.7 ± 6.7 | .33 |
| Wt loss, % of body wt, mean ± SD | 19.9 ± 10.4 | 22.4 ± 8.6 | .16 |
| Wt loss, % mBMI, mean ± SD | 33.8 ± 25.9 | 27.9 ± 15.5 | .13 |
| Duration of wt loss, mo, mean ± SD | 15.4 ± 15.4 | 16.2 ± 18.7 | .79 |
| Rate of loss, kg per mo, mean ± SD | 1.6 ± 1.3 | 1.6 ± 1.4 | .95 |
| Rate of % mBMI loss, % per mo, mean ± SD | 3.6 ± 2.6 | 3.5 ± 3.1 | .84 |
| Duration of amenorrhea, mo,a mean ± SD | 6.7 ± 5.3 | 10.5 ± 9.3 | .10 |
| No menses in 3 mo,a % (n) | 77 (20) | 86 (30) | .81 |
| ED psychopathology, EDE-Q score, mean ± SD | n = 42 | n = 58 | |
| Global score | 3.80 ± 1.66 | 3.00 ± 1.66 | .02 |
| Wt concerns | 3.75 ± 1.87 | 2.83 ± 1.82 | .02 |
| Shape concerns | 4.35 ± 1.89 | 3.49 ± 1.77 | .02 |
| Restraint | 4.00 ± 1.79 | 3.09 ± 2.01 | .02 |
| Eating concerns | 3.10 ± 1.65 | 2.59 ± 1.54 | .12 |
| Admission wt and vital signs, mean ± SD | |||
| Admission % mBMI | 95.2 ± 9.0 | 76.5 ± 5.9 | <.001 |
| Lowest 24-h HR, beats per min | 40.3 ± 4.7 | 42.0 ± 7.0 | .15 |
| Lowest SBP, mmHg | 96.4 ± 8.1 | 92.0 ± 7.6 | .004 |
| Lowest temperature, °C | 36.6 ± 0.1 | 36.6 ± 0.1 | .99 |
| Orthostatic change in HR, increase ≥20 beats per min | 25.8 ± 14.9 | 26.7 ± 12.5 | .74 |
| Orthostatic change in systolic BP, decrease ≥10 mmHg | 1.3 ± 8.5 | 0.6 ± 5.8 | .63 |
| Percent with vital sign instabilities, % (n) | |||
| Bradycardia, <50 beats per min | 96 (48) | 89 (59) | .17 |
| Hypotension, systolic BP <90 mmHg | 18 (9) | 41 (27) | .009 |
| Hypothermia, <36.0°C | 0 (0) | 2 (1) | .57 |
| Orthostatic HR, increase ≥20 beats per min | 54 (27) | 65 (43) | .23 |
| Orthostatic systolic BP, decrease ≥10 mmHg | 14 (7) | 6 (4) | .15 |
| Admission laboratory values (reference range), mean ± SD | |||
| Creatinine (0.50–0.80 mg/dL) | 0.74 ± 0.10 | 0.69 ± 0.11 | .08 |
| AST (25–40 IU/L) | 25.7 ± 12.5 | 26.6 ± 12.3 | .72 |
| ALT (20–50 IU/L) | 23.9 ± 12.1 | 33.1 ± 26.7 | .03 |
| Phosphorus (3.0–5.1 mg/dL) | 3.8 ± 0.6 | 3.7 ± 0.5 | .48 |
| Magnesium (1.8–2.4 mg/dL) | 2.1 ± 0.2 | 2.2 ± 0.1 | .27 |
| Potassium (3.5–5.1 mmol/L) | 3.8 ± 0.3 | 3.8 ± 0.4 | .78 |
| Percent with laboratory abnormalities, % (n) | |||
| High creatinine, >0.8 mg/dL | 36 (18) | 23 (15) | .78 |
| High AST, >40 IU/L | 6 (3) | 12 (8) | .59 |
| High ALT, >30 IU/L7 | 20 (10) | 33 (22) | .90 |
| Hypophosphatemia, <3.0 mg/dL3 | 6 (3) | 6 (4) | .65 |
| Hypokalemia, <3.5 mmol/L | 14 (7) | 14 (9) | .58 |
| Hypomagnesemia, <1.8 mg/dL | 8 (4) | 0 (0) | .03 |
The values presented as mean ± SD have P values from independent 2-sample t tests; values presented as % (n) have P values from Fisher’s exact tests. Reference ranges are taken from the Clinical Laboratory Improvement Amendment laboratory manual for adolescents unless otherwise noted.
Among postmenarcheal girls with ≥ 3 mo since their last menstrual period and who are not taking hormonal contraception.
Results
Description and Comparison of Participants With AAN and AN
A total of 116 participants enrolled: 50 with AAN and 66 with AN. Table 1 compares the AAN and AN groups on admission. The study population did not differ from the wider clinical inpatient ED population by key demographic characteristics, including age, sex, and race and/or ethnicity.
Demographics
Participants were 16.5 ± 2.6 years old on average, 91% were of female sex. Participants were predominantly non-Hispanic white and Asian American and of “average” SES (defined as occupational score >4.0),24 with no significant differences seen between groups.
Weight and Menstrual History
Historical BMI (25.2 ± 5.6 vs 20.7 ± 3.0; P < .001) and percentage of mBMI (129.2 ± 28.8 vs 104.3 ± 16.9; P < .001) were higher in AAN than AN. There were no significant differences in weight history or duration of amenorrhea. Absolute weight loss was ∼13.5 kg over a duration of ∼15.9 months at a rate of 1.6 kg per month or 3.5% per month.
ED Psychopathology
Psychological symptoms were significantly greater in AAN than in AN (3.80 ± 1.66 vs 3.00 ± 1.66; P = .02), with a mean EDE-Q global score in AAN >1 SD above the community mean of 3.38.25 All subscale scores except eating concerns were higher in AAN (all P < .05).
Admission Weight, Vital Signs and Laboratory Values
By definition, participants with AAN had a significantly higher admission percentage of mBMI than those with AN (95.2 ± 9.0 vs 76.5 ± 5.9%; P < .001). There was no difference in the lowest HR between groups (40.3 ± 4.7 vs 42.0 ± 7.0 beats per minute; P = .15); 93% had an HR <50 beats per minute. Those with AAN had higher systolic BP (96.4 ± 8.1 vs 92.0 ± 7.6 mmHg; P = .004) and were less likely to present with hypotension (systolic BP <90 mmHg; 18% vs 41%, respectively; P = .009). Alanine aminotransferase (ALT) was lower in AAN (23.9 ± 12.1 vs 33.1 ± 26.7 IU/L; P = .03). There were no differences in serum electrolytes.
Association Between Individual Weight History Variables and Presentation Characteristics
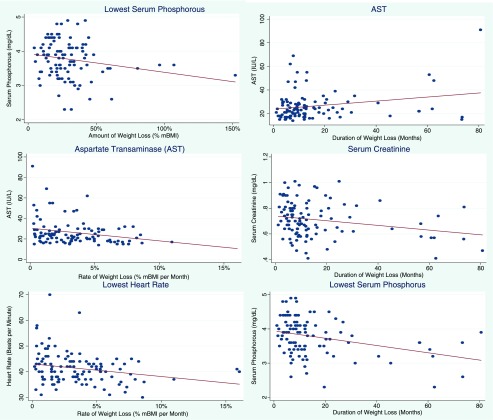
Table 2 shows associations between 3 weight variables and illness severity. Bivariate associations with admission percentage of mBMI and markers of illness severity are shown in the first column (model 0). Higher admission percentage of mBMI was associated with lower HR, higher BP, lower ALT, higher serum phosphorus, and higher (worse) EDE-Q global scores (β = 0.042 [CI 0.013 to 0.070]; P = .004) and all subscale scores except eating concerns. Associations between amount of loss (model 1), rate of loss (model 2), and duration of loss (model 3) and markers of illness severity are shown, unadjusted and adjusted for admission percentage of mBMI. Significant findings from these models are also depicted in Fig 2 (scatter plots with dots showing the unadjusted relationship and a line adjusted for admission percentage of mBMI): greater percentage of mBMI loss and lower serum phosphorus (β = −0.005 [confidence interval (CI) −0.001 to −0.000]; P = .04); faster rate of percentage of mBMI loss and lower HR (β = −0.492 [CI −0.883 to −0.100]; P = .01) and lower aspartate transaminase (AST) (β= −1.24 [CI −2.25 to −0.228]; P = .02); longer duration of weight loss and lower creatinine (β = −0.002 [CI −0.003 to −0.000]; P = .02); higher AST (β = 0.171 [CI −0.031 to −0.312]; P = .02); and lower serum phosphorus (β = −0.011 [CI −0.017 to 0.005]; P = .001).
TABLE 2.
Associations Between Admission Wt, Amount of Loss, Rate of Loss, and Duration of Loss and Markers of Illness Severity
| Markers of Illness Severity | Model 0 (Admission), % mBMI | Model 1 (Amount of Loss), % mBMI | Model 2 (Rate of Loss), % mBMI per mo | Model 3 (Duration of Loss), mo | |||
|---|---|---|---|---|---|---|---|
| Unadjusted | Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| β (CI) | β (CI) | β (CI) | β (CI) | β (CI) | β (CI) | β (CI) | |
| EDE-Q scores | |||||||
| Global score | .042** (0.013 to 0.070) | .011 (−0.005 to 0.026) | .007 (−0.008 to 0.023) | .036 (−0.091 to 0.163) | −.003 (−0.129 to 0.122) | .012 (−0.007 to 0.031) | .017 (−0.002 to 0.034) |
| Wt concerns | .078** (0.017 to 0.079) | .014 (−0.007 to 0.028) | .006 (−0.011 to 0.023) | .047 (−0.094 to 0.188) | .003 (−0.137 to 0.142) | .010 (−0.011 to 0.031) | .015 (−0.005 to 0.035) |
| Shape concerns | .042 (0.011 to 0.073) | .011 (−0.006 to 0.028) | .007 (−0.010 to 0.024) | .019 (−0.129 to 0.157) | −.023 (−0.163 to 0.117) | .014 (−0.006 to 0.034) | .018 (0.001 to 0.039) |
| Restraint | .050** (0.017 to 0.082) | .017 (−0.001 to 0.035) | .014 (−0.005 to 0.030) | −.055 (−0.009 to 0.200) | .001 (−0.134 to 0.154) | .016 (−0.006 to 0.037) | .021 (0.000 to 0.041) |
| Eating concerns | .027 (0.000 to 0.054) | .005 (−0.010 to 0.020) | .003 (−0.012 to 0.018) | .022 (−0.098 to 0.142) | −.004 (−0.125 to 0.118) | .008 (−0.009 to 0.026) | .011 (−0.006 to 0.029) |
| Vital signs | |||||||
| Lowest 24-h HR, beats per min | −.099* (−0.193 to −0.004) | −.027 (−0.082 to 0.028) | −.019 (−0.074 to 0.036) | −.535 (−0.927 to −0.142) | −.492* (−0.883 to −0.100) | .055 (−0.011 to 0.121) | .048 (−0.018 to 0.113) |
| Systolic BP, mmHg | .171 (0.049 to 0.294) | −.282 (−0.101 to 0.044) | −.043 (−0.114 to 0.028) | .090 (−0.437 to 0.617) | .002 (−0.514 to 0.510) | −.028 (−0.115 to 0.059) | −.014 (−0.099 to 0.071) |
| Laboratory values | |||||||
| Creatinine, mg/dL | .001 (−0.001 to 0.003) | −.000 (−0.001 to 0.001) | −.000 (−0.001 to 0.001) | .006 (−0.004 to 0.002) | .005 (−0.005 to 0.001) | −.001* (−0.003 to 0.000) | −.002* (−0.003 to −0.000) |
| AST, IU/L | −.186 (−0.383 to −0.010) | −.061 (−0.182 to 0.059) | −.040 (−0.162 to 0.082) | −1.42** (−2.41 to 0.439) | −1.24* (−2.25 to 0.228) | .186* (0.045 to 0.377) | .171* (0.031 to 0.312) |
| ALT, IU/L | −.382 (−0.731 to 0.032) | .002 (−0.198 to .227) | −.069 (−0.149 to 0.287) | .048 (−1.79 to 1.89) | .595 (−1.27 to 2.46) | −.053 (−0.316 to 0.209) | −.088 (−0.347 to 0.170) |
| Phosphorus, mg/dL | .001* (0.001 to 0.0002) | −.005 (−0.010 to 0.001) | −.005* (−0.010 to 0.000) | .004 (−0.004 to 0.008) | .003 (−0.001 to 0.007) | −.011 (−0.018 to −0.005) | −.011** (−0.017 to −0.005) |
| Potassium, mmol/L | −.001 (−0.006 to 0.005) | −.003 (−0.006 to 0.000) | −.003 (−0.007 to 0.000) | −.008 (−0.003 to 0.002) | −.007 (−0.003 to 0.002) | −.003 (−0.007 to 0.001) | −.003 (−0.007 to −0.001) |
| Magnesium, mg/dL | −.002 (−0.005 to 0.001) | .000 (−0.001 to 0.002) | .000 (−0.001 to 0.002) | .010 (−0.002 to 0.002) | .001* (−0.000 to 0.024) | −.000 (−0.002 to 0.001) | −.000 (−0.002 to 0.001) |
Multivariable model adjusted for admission percentage of mBMI.
P < .05; ** P < .01.
FIGURE 2.
Significant associations between weight history variables and markers of illness severity. Dots depict the unadjusted association between the weight loss variable and marker of illness severity for each participant. The line illustrates the predicted relationship from multivariable linear regression adjusted for admission percentage of mBMI. β coefficients with 95% CIs, P values, and adjusted R2 from adjusted multivariable linear regression are shown. Findings were as follows. Lowest serum phosphorous (amount): β = −.005 (95% CI −.010 to .000); P = .04; adjusted R2 = 0.074. AST (duration): β = .171 (95% CI .031 to .312); P = .02; adjusted R2 = 0.076. AST (rate): β = −1.24 (95% CI −2.25 to .228); P = .02; adjusted R2 = 0.076. Serum creatinine: β = −.002 (95% CI −.003 to −.000); P = .01; adjusted R2 = 0.044. Lowest HR: β = −.492 (95% CI −.883 to −.100); P = .01; adjusted R2 = 0.070. Lowest serum phosphorous (duration): β = −.011 (95% CI −.017 to−.005); P = .001; adjusted R2 = 0.044.
Predictive Value of Comprehensive Weight History on Key Indicators of Severity of Illness
Table 3 illustrates the results of stepwise regression analyses.
TABLE 3.
Results of Stepwise Multivariable Linear Regression Analysis With Backward Selection
| Serum Phosphorus | HR | EDE-Q Global Score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | 95% CI | P | β | SE | 95% CI | P | β | SE | 95% CI | P | ||
| Full study populationa | |||||||||||||
| High historical % mBMI | .004 | 0.003 | (−0.002 to 0.009) | .17 | −.063 | 0.049 | (−0.160 to 0.033) | .20 | −.657 | 0.336 | (−1.32 to 0.010) | .05 | |
| High historical BMI | — | — | — | — | — | — | — | — | −.360 | 0.166 | (−0.691 to −0.030) | .03 | |
| Age, y | — | — | — | — | .709 | 0.235 | (0.244 to 1.17) | .003 | .355 | 0.094 | (0.169 to 0.542) | <.001 | |
| Admission % mBMI | — | — | — | — | — | — | — | — | .780 | 0.334 | (0.117 to 1.44) | .02 | |
| Wt loss, % mBMI | — | — | — | — | .153 | 0.084 | (−0.013 to 0.318) | .07 | .728 | 0.333 | (0.066 to 1.38) | .03 | |
| Wt loss, % body wt | −.020 | 0.007 | (−0.033 to −0.006) | .006 | — | — | — | — | — | — | — | — | |
| Wt loss, kg | — | — | — | — | −.286 | 0.143 | (−0.570 to −0.002) | .05 | — | — | — | — | |
| Duration wt loss, mo | −.012 | 0.003 | (−0.017 to −0.005) | <.001 | — | — | — | — | — | — | — | — | |
| Rate of loss, % mBMI | — | — | — | — | — | — | — | — | — | — | — | — | |
| Rate of loss, kg per mo | — | — | — | — | — | — | — | — | — | — | — | — | |
| Final model | R2 = 0.189 | R2 = 0.126 | R2 = 0.243 | ||||||||||
| Adjusted R2 = 0.164 | Adjusted R2 = 0.093 | Adjusted R2 = 0.202 | |||||||||||
| Among girls with amenorrheab | |||||||||||||
| High historical BMI | — | — | — | — | −1.74 | 0.74 | (−3.24 to −0.24) | .02 | −.580 | 0.292 | (−1.17 to 0.11) | .05 | |
| High historical % mBMI | 3.29 | 1.13 | (0.990 to 5.60) | .006 | −11.2 | 8.23 | (−27.8 to 5.42) | .18 | .107 | 0.056 | (−0.557 to 0.220) | .06 | |
| Age, y | −.118 | 0.038 | (−0.195 to −0.040) | .004 | 1.72 | 0.44 | (0.841 to 2.50) | <.001 | .428 | 0.183 | (0.057 to 0.800) | .02 | |
| Wt loss, % mBMI | −3.27 | 1.13 | (−5.57 to −0.977) | .007 | 11.5 | 8.25 | (−5.16 to 28.1) | .17 | — | — | — | — | |
| Admission % mBMI | −3.30 | 1.14 | (−5.61 to −0.988) | .006 | 11.5 | 8.25 | (−5.17 to 28.1) | .17 | — | — | — | — | |
| Amenorrhea, mob | .022 | 0.012 | (−0.002 to 0.046) | .07 | −.111 | 0.081 | (−0.276 to 0.054) | .18 | −.071 | 0.030 | (−0.132 to −0.010) | .02 | |
| Wt loss, % body wt | −.076 | 0.026 | (−0.129 to −0.022) | .007 | — | — | — | — | — | — | — | — | |
| Wt loss, kg | — | — | — | — | — | — | — | — | — | — | — | — | |
| Duration wt loss, mo | — | — | — | — | — | — | — | — | — | — | — | — | |
| Rate of loss, % mBMI | −.340 | 0.133 | (−0.610 to −0.071) | <.001 | — | — | — | — | — | — | — | — | |
| Rate of loss, kg per mo | .950 | 0.310 | (0.331 to 1.58) | .004 | — | — | — | — | — | — | — | — | |
| Final model | R2 = 0.477 | R2 = 0.367 | R2 = 0.174 | ||||||||||
| Adjusted R2 = 0.358 | Adjusted R2 = 0.278 | Adjusted R2 = 0.091 | |||||||||||
—, removed from model during backward selection at significance level of 0.20.
Ten variables were entered into the model (all 8 wt history variables, age, and admission percentage of mBMI); β coefficients, SEs, 95% CIs, and P values are shown for all variables that contributed to the best fit with R2 and adjusted R2 for final model. If no data are shown for a given variable, that variable was removed from the model in backward selection with α = .20.
Eleven variables were entered into the model: all 8 wt history variables, age, and admission percentage of mBMI plus duration of amenorrhea. This model included 50 postmenarcheal girls with ≥ 3 mo since their last menstrual period and who were not taking hormonal contraception.
Serum Phosphorus
In the weight history model, 3 variables contributed significantly to the fit of the final model for serum phosphorus: highest historical BMI, percentage of body weight loss, and duration of weight loss (in months). The adjusted R2 for the final model was 0.164. In the weight and menstrual history model, 8 variables contributed significantly to the fit: highest historical BMI, age, amount of weight loss (percentage of mBMI loss and percentage of body weight loss), admission percentage of mBMI, duration of amenorrhea, and rate of loss (percentage of mBMI loss per month and kilograms lost per month). The adjusted R2 for the final model was 0.358.
HR
The weight history model retained 4 variables: highest historical percentage of mBMI, age, and amount of weight lost (percentage of mBMI loss and kilograms lost). The adjusted R2 was 0.093. The weight and menstrual history model retained 6 variables: highest historical percentage of mBMI, BMI, age, amount of percentage of mBMI loss and kilograms lost, and duration of amenorrhea. The final model adjusted R2 was 0.278.
EDE-Q Global Score
The weight history model retained 5 variables: highest historical percentage of mBMI, BMI, age, admission percentage of mBMI, and amount of percentage of mBMI loss. The final model adjusted R2 was 0.202. The weight and menstrual history model retained 4 variables: highest historical percentage of mBMI, BMI, age, and duration of amenorrhea. The final model adjusted R2 was 0.091.
Discussion
Adolescents admitted to the hospital with AAN or AN who experienced a greater amount, rate, or duration of weight loss had significantly worse medical and nutritional status independent of admission weight. These findings build on 1 previous study of WS in AAN, in which a greater amount and recency of weight loss predicted lower HR.14 The marked rise in adolescents with AAN requiring hospitalization at apparently normal weights13–15 has challenged the historical reliance on current weight to guide clinical concern. Bradycardia is a primary indicator for medical hospitalization,21 and cardiac arrhythmia with electrolyte disturbances contribute to increased mortality in AN.27 In the current study population, faster rate of weight loss before hospitalization predicted lower HR at admission across both diagnoses and all body weights. This finding supports the need for early intervention regardless of body weight.
Numerous hormonal and metabolic adaptations to starvation were documented in weight-reduced subjects with obesity28,29 and low-weight patients with AN as long as 20 years ago.30 More recently, WS has gained traction as a potential marker of illness severity18 and predictor of treatment outcomes in adults with AN.19 Before the recognition of AAN, studies including normal-weight patients with ED reported lower HR in those with larger amounts of 16 or faster weight losses.17 Whitelaw et al14 recently confirmed this in patients with AAN. Contrary to their results, we did not find a relationship between WS and HR, although we did find a robust association with rate of weight loss: for every 2% mBMI per month faster loss, HR was 1 beat per minute lower on admission. These findings suggest that HR is sensitive to acute nutritional insult even if weight remains normal.
The timing of malnutrition is salient in adolescents because of the advanced nutritional demands of growth and puberty.31 We found that participants with AAN were just as likely to have amenorrhea as those with AN. One study identified WS as a predictor of prolonged amenorrhea during follow-up of formerly overweight patients with ED,22 suggesting that hypothalamic-pituitary-gonadal suppression due to malnutrition in AN30 can also result from WS. We examined the explanatory value of weight history on illness severity with and without the menstrual history. For HR, the variance explained by weight history increased dramatically from 9% to 28% when duration of amenorrhea was added. For serum phosphorus, model fit improved from 16% to 36%. For context, stepwise regression models of powerful cardiovascular risk factors (eg, smoking) explain 52% of the variance in disease outcomes.32 Thus, among postmenarcheal girls, weight and menstrual history contribute meaningfully to the assessment of medical and nutrition status. The interactive effects of weight history and pubertal stage on medical and nutritional status require further investigation.
Our main finding related to WS is that participants who had lost greater amounts had lower serum phosphorus on admission regardless of admission weight. The possibility that normal-weight patients could be at increased risk during refeeding challenges the historical thinking that low-weight patients warranted the most concern.3–6 Although our study was limited to baseline, Whitelaw et al14 followed patients during refeeding and found that greater WS predicted lower phosphate nadir. Together these findings support consideration of WS to determine which patients may need more cautious refeeding or intensive medical monitoring. For decades, lower-calorie refeeding was applied broadly to ensure the safety of all patients.33 However, this approach is linked to poor weight gain and protracted hospital stay,9,10,12 and clinical practice is moving toward more aggressive refeeding. The need for new tools to assess risk in today’s inpatient population with diverse diagnoses and body weights13–15 is pressing.
ED psychopathology was significantly worse in AAN, in accordance with Sawyer et al.15 Higher EDE-Q scores were consistently associated with higher admission weight but not weight history. This had not been previously examined in adolescents, aside from a subanalysis by Whitelaw et al.14 The lack of association was striking given the relationship between greater WS and worse ED psychopathology in the adult literature.18,19 In stepwise regression, weight history explained 20% of the variance in EDE-Q global scores, and model fit was reduced by adding amenorrhea, suggesting that other factors powerfully influence ED psychopathology in postmenarcheal girls. Nevertheless, the reproducible finding that patients with AAN have worse ED psychopathology continues to dispel the misconception that AAN is a lesser illness than AN and heightens the need to examine whether there are ED cognitions or behaviors that are unique to AAN.
Duration of weight loss had not been previously examined in AAN. It is clear that severity increases with duration of AN,1,2 underscoring the importance of early intervention. A longer duration of weight loss was associated with lower serum phosphorus, lower creatinine, and higher AST regardless admission weight. As to how these markers may indicate nutritional status, serum creatinine is decreased in malnutrition related to lean mass wasting,34 which contributes to hypophosphatemia.35 Although creatinine must be interpreted with caution in AN,36 our findings suggest that relative reductions in lean mass are detrimental to nutritional status even if total lean mass remains normal, as shown in AAN.37 Aminotransferases were of interest as markers of malnutrition; they are elevated in roughly one-third of patients with AN7 and rise in proportion to lower weight7,38 and fat mass.7 Accordingly, we found elevated ALT in 30% of participants with AN, increasing in proportion to lower weight. ALT was significantly lower in the AAN group but increased with longer duration, suggesting that the relatively higher fat mass documented in AAN37 is hepatoprotective during early weight loss. Studies should examine whether there are tissue-specific effects of weight loss related to body composition.
We acknowledge several limitations. First, patients at <60% mBMI were excluded; findings cannot be generalized to patients of extremely low weight. Second, we do not report medication and alcohol use, which could impact measures such aminotransferases.7 Third, examinations of menstrual status were limited to the 43% of participants who were postmenarcheal girls. Fourth, these baseline data cannot be extrapolated to inform risk during refeeding. Fifth, although self-reported weight is more accurate in AN than in other diagnoses,39 it is less reliable than objective measurement. Finally, participants were already identified or referred to our ED specialty programs and subsequently hospitalized, making measures such as telemetry feasible. However, weight histories were collected by questionnaire and could be performed by pediatricians in any clinical setting to help guide clinical concern and make treatment decisions (eg, whether to refer for specialty care).
Conclusions
Assessment of patients with AN has traditionally relied on current weight, with the highest index of concern being for low-weight patients. Our findings support concern for patients with large, fast, and/or long-duration weight loss even if current weight remains normal. Future research is needed to examine the use of weight history to inform treatment decisions and outcomes.
Acknowledgments
We thank the patients who participated in this project and Anna Krinkle for her assistance with database creation and management.
Glossary
- AAN
atypical anorexia nervosa
- ALT
alanine aminotransferase
- AN
anorexia nervosa
- AST
aspartate transaminase
- BP
blood pressure
- CI
confidence interval
- ED
eating disorder
- EDE-Q
Eating Disorder Examination Questionnaire
- HR
heart rate
- mBMI
median BMI
- SES
socioeconomic status
- WS
weight suppression
Footnotes
Deidentified individual participant data will not be made available.
Dr Garber is a principal investigator on this project, developed the original idea, conceptualized and designed the project, performed the data analysis, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Golden is a principal investigator on this project and assisted with the development of the original idea, conceptualization and design of the project, data analysis, drafting of the initial manuscript, and review and revision of the manuscript; Dr Cheng is the faculty biostatistician and oversaw the Data Coordination Center, which managed the data collection from clinical sites and developed the analytic plan; Dr Adams is the lead data analyst at the Data Coordination Center; Ms Machen is the senior research associate at the Data Coordination Center; Ms Saffran and Ms Kreiter were research assistants, collected data, and reported to the Data Coordination Center; Drs Buckelew, Kapphahn, and Moscicki and Ms Sy enrolled participants or advised on clinical care for study participants; Drs Accurso and Le Grange oversaw the psychometric data collection and critically reviewed the manuscript for psychological content; Dr Wilson contributed to the questionnaire design and implementation; and all authors read, revised, and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT02488109).
FINANCIAL DISCLOSURE: Dr Le Grange receives royalties from Guilford Press and Routledge and is a codirector of the Training Institute for Child and Adolescent Eating Disorders, LLC; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health (R01HD082166). Dr Garber’s time was also supported in part by the Health Resources and Services Administration leadership training in adolescent health (T71MC00003). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Miller KK, Grinspoon SK, Ciampa J, et al. . Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165(5):561–566 [DOI] [PubMed] [Google Scholar]
- 2.Misra M, Aggarwal A, Miller KK, et al. . Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;114(6):1574–1583 [DOI] [PubMed] [Google Scholar]
- 3.Ornstein RM, Golden NH, Jacobson MS, Shenker IR. Hypophosphatemia during nutritional rehabilitation in anorexia nervosa: implications for refeeding and monitoring. J Adolesc Health. 2003;32(1):83–88 [DOI] [PubMed] [Google Scholar]
- 4.O’Connor G, Nicholls D. Refeeding hypophosphatemia in adolescents with anorexia nervosa: a systematic review. Nutr Clin Pract. 2013;28(3):358–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Society for Adolescent Health and Medicine Refeeding hypophosphatemia in hospitalized adolescents with anorexia nervosa: a position statement of the Society for Adolescent Health and Medicine. J Adolesc Health. 2014;55(3):455–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CA, Sabel AL, Gaudiani JL, Mehler PS. Predictors of hypophosphatemia during refeeding of patients with severe anorexia nervosa. Int J Eat Disord. 2015;48(7):898–904 [DOI] [PubMed] [Google Scholar]
- 7.Fong HF, Divasta AD, Difabio D, et al. . Prevalence and predictors of abnormal liver enzymes in young women with anorexia nervosa. J Pediatr. 2008;153(2):247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes EK, Le Grange D, Court A, Sawyer SM. A case series of family-based treatment for adolescents with atypical anorexia nervosa. Int J Eat Disord. 2017;50(4):424–432 [DOI] [PubMed] [Google Scholar]
- 9.Garber AK, Mauldin K, Michihata N, et al. . Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized adolescents with anorexia nervosa. J Adolesc Health. 2013;53(5):579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garber AK, Michihata N, Hetnal K, Shafer MA, Moscicki AB. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J Adolesc Health. 2012;50(1):24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garber AK, Sawyer SM, Golden NH, et al. . A systematic review of approaches to refeeding in patients with anorexia nervosa. Int J Eat Disord. 2016;49(3):293–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden NH, Keane-Miller C, Sainani KL, Kapphahn CJ. Higher caloric intake in hospitalized adolescents with anorexia nervosa is associated with reduced length of stay and no increased rate of refeeding syndrome. J Adolesc Health. 2013;53(5):573–578 [DOI] [PubMed] [Google Scholar]
- 13.Whitelaw M, Gilbertson H, Lee KJ, Sawyer SM. Restrictive eating disorders among adolescent inpatients. Pediatrics. 2014;134(3). Available at: www.pediatrics.org/cgi/content/full/134/3/e758 [DOI] [PubMed] [Google Scholar]
- 14.Whitelaw M, Lee KJ, Gilbertson H, Sawyer SM. Predictors of complications in anorexia nervosa and atypical anorexia nervosa: degree of underweight or extent and recency of weight loss? J Adolesc Health. 2018;63(6):717–723 [DOI] [PubMed] [Google Scholar]
- 15.Sawyer SM, Whitelaw M, Le Grange D, Yeo M, Hughes EK. Physical and psychological morbidity in adolescents with atypical anorexia nervosa. Pediatrics. 2016;137(4):e20154080. [DOI] [PubMed] [Google Scholar]
- 16.Peebles R, Hardy KK, Wilson JL, Lock JD. Are diagnostic criteria for eating disorders markers of medical severity? Pediatrics. 2010;125(5). Available at: www.pediatrics.org/cgi/content/full/125/5/e1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swenne I. Influence of premorbid BMI on clinical characteristics at presentation of adolescent girls with eating disorders. BMC Psychiatry. 2016;16:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berner LA, Shaw JA, Witt AA, Lowe MR. The relation of weight suppression and body mass index to symptomatology and treatment response in anorexia nervosa. J Abnorm Psychol. 2013;122(3):694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wildes JE, Marcus MD. Weight suppression as a predictor of weight gain and response to intensive behavioral treatment in patients with anorexia nervosa. Behav Res Ther. 2012;50(4):266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors . Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr. 2012;36(3):275–283 [DOI] [PubMed] [Google Scholar]
- 21.Golden NH, Katzman DK, Sawyer SM, et al. ; Society for Adolescent Health and Medicine . Position paper of the Society for Adolescent Health and Medicine: medical management of restrictive eating disorders in adolescents and young adults. J Adolesc Health. 2015;56(1):121–125 [DOI] [PubMed] [Google Scholar]
- 22.Seetharaman S, Golden NH, Halpern-Felsher B, et al. . Effect of a prior history of overweight on return of menses in adolescents with eating disorders. J Adolesc Health. 2017;60(4):469–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Clinical growth charts. 2000. Available at: https://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed on June 30, 2019
- 24.Cirino PT, Chin CE, Sevcik RA, et al. . Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 2002;9(2):145–155 [DOI] [PubMed] [Google Scholar]
- 25.Fairburn CG, Cooper Z, O’Connor ME. Eating Disorder Examination (Edition 16.0D) In: Fairburn CG, ed. Cognitive Behavior Therapy and Eating Disorders. New York, NY: Guilford Press; 2008:265–308 [Google Scholar]
- 26.Nachtstem CJ, Neter J, Kutner MH. Applied Linear Regression Models, 4th ed Blacklick, OH: McGraw-Hill Education; 2004:289 [Google Scholar]
- 27.Portilla MG. Bradycardia: an important physical finding in anorexia nervosa. J Ark Med Soc. 2011;107(10):206–208 [PubMed] [Google Scholar]
- 28.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628 [DOI] [PubMed] [Google Scholar]
- 29.Douyon L, Schteingart DE. Effect of obesity and starvation on thyroid hormone, growth hormone, and cortisol secretion. Endocrinol Metab Clin North Am. 2002;31(1):173–189 [DOI] [PubMed] [Google Scholar]
- 30.Golden NH, Shenker IR. Amenorrhea in anorexia nervosa. Neuroendocrine control of hypothalamic dysfunction. Int J Eat Disord. 1994;16(1):53–60 [DOI] [PubMed] [Google Scholar]
- 31.Soliman A, De Sanctis V, Elalaily R. Nutrition and pubertal development. Indian J Endocrinol Metab. 2014;18(suppl 1):S39–S47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanktree MB, Hegele RA, Schork NJ, Spence JD. Extremes of unexplained variation as a phenotype: an efficient approach for genome-wide association studies of cardiovascular disease. Circ Cardiovasc Genet. 2010;3(2):215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Psychiatric Association Work Group on Eating Disorders Practice guideline for the treatment of patients with eating disorders (revision). American Psychiatric Association Work Group on Eating Disorders. Am J Psychiatry. 2000;157(suppl 1):1–39 [PubMed] [Google Scholar]
- 34.Hari P, Bagga A, Mahajan P, Lakshmy R. Effect of malnutrition on serum creatinine and cystatin C levels. Pediatr Nephrol. 2007;22(10):1757–1761 [DOI] [PubMed] [Google Scholar]
- 35.Håglin L. Hypophosphataemia in anorexia nervosa. Postgrad Med J. 2001;77(907):305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mira M, Stewart PM, Vizzard J, Abraham S. Biochemical abnormalities in anorexia nervosa and bulimia. Ann Clin Biochem. 1987;24(pt 1):29–35 [DOI] [PubMed] [Google Scholar]
- 37.Nagata JM, Carlson JL, Golden NH, et al. . Comparisons of bone density and body composition among adolescents with anorexia nervosa and atypical anorexia nervosa. Int J Eat Disord. 2019;52(5):591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata JM, Park KT, Colditz K, Golden NH. Associations of elevated liver enzymes among hospitalized adolescents with anorexia nervosa. J Pediatr. 2015;166(2):439–443.e1 [DOI] [PubMed] [Google Scholar]
- 39.McCabe RE, McFarlane T, Polivy J, Olmsted MP. Eating disorders, dieting, and the accuracy of self-reported weight. Int J Eat Disord. 2001;29(1):59–64 [DOI] [PubMed] [Google Scholar]