Abstract
Purpose
Controversies exist for which treatment is optimal for early hepatocellular carcinoma (HCC): radiofrequency ablation (RFA), surgical resection (SR), or transplantation (LT). We compared outcomes between treatments as first-line therapy for HCC patients measuring up to 5 cm or different cancer risk groups.
Patients and methods
The Surveillance, Epidemiology, and End Results database was retrieved for HCC patients treated with RFA, SR, or LT between 2004 and 2015. The effects of three treatments were compared using propensity score, inverse probability of treatment weights adjustment, and instrumental variable analysis for overall survival (OS) and competing risks regression models for disease-specific survival (DSS). We also evaluated whether the effect of treatments varied according to baseline clinical characteristics by locally weighted regression method.
Results
Of 7664 patients, RFA and SR yielded worse OS (HR 1.67, CI 1.43–1.70, P<0.001; HR 1.43, CI 1.40–1.67, P<0.001) and DSS (HR 2.00, CI 1.10–3.30, P<0.011; HR 2.50, CI 2.00–3.30, P<0.001) than LT. In patients with small tumors, SR may confer more survival benefits than RFA (HR>1) for different tumor sizes measuring up to 5 cm and may be an appropriate first-line treatment. Additionally, RFA has more survival benefits compared with SR (HR 0.83, CI 0.53–1.25) for those patients with low tumor risk and good general health condition (without any prognostic risk factors). However, those patients with a predicted 5-year overall mortality risk >30% seem to benefit more for SR than RFA.
Conclusion
Due to a shortage of donors, RFA and SR can be applied as either primary management of HCC or as a bridging therapy for LT. Furthermore, SR is an effective option for patients with different HCC tumor size. However, RFA could achieve comparable survival benefits with SR for patients without any risk factors.
Keywords: hepatocellular carcinoma, outcomes, radiofrequency ablation, surgical resection, transplantation, propensity score, PS
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of all cancer-specific deaths and the sixth most common cancer worldwide.1–4 HCC patients were classified by the current Barcelona Clinic for Liver Cancer (BCLC) staging system with single tumors <5 cm or no more than 3 tumors each <3 cm in diameter without major vascular invasion and metastasis as very-early-stage and early-stage HCC. HCC patients were recommended by liver transplantation (LT), surgical resection (SR), and radiofrequency ablation (RFA) as treatment types.4,5
LT is a great curative treatment option for HCC patients amenable to transplantation, addressing both the underlying liver disease as well as the malignant lesions.6 Unfortunately, due to the significant donor shortage, LT is not a feasible option for the majority of HCC patients. In addition, most HCC patients are not candidates for SR when diagnosed at the first time for various reasons, including extrahepatic metastasis, vascular invasion, large tumor size, large number of lesions, or comorbid conditions.7 Although treatment techniques such as portal vein embolization have been developed to increase the chances of successful SR, inadequate future liver remnant within the underlying liver disease and vascular invasion still represents a significant barrier to SR.8,9 RFA also has been accepted as an adequate and widely used option for early HCC patients.10
While for HCC patients who are candidates for RFA, SR, and LT, significant controversies still exist, regarding to which treatment provides our best clinical outcomes. Many recent studies comparing RFA with SR have revealed comparable clinical outcomes for RFA in patients with early-stage HCC (tumor size of 2–5 cm).11–13 Although RFA can be excellent as first-line treatment, the maximum HCC tumor size for which RFA is safe and effective still remains controversial; the size cutoff values of 20 mm, 30 mm, and 50 mm have been proposed recently.11,13 A 20-mm cutoff value based on studies has been proposed demonstrating that SR causing longer survival than RFA group in patients with poorly differentiated HCC measuring <20 mm.14 Although concerns that RFA is lack of efficiency for lesions measuring >30 mm, some reports have demonstrated that lesions measuring up to 50 mm can be ablated safely.15–17 In order to address this controversy, recent study18 have been performed and demonstrated that RFA offered worse results than SR or LT for HCC tumors measuring >30 mm. Above prior studies concluded results in choosing optimal treatments for early-stage HCC by using Surveillance, Epidemiology, and End Results (SEER) database; however, some inadequate methodological limitations, such as measurable or unmeasurable confounders affecting groups based on the stratification by age or tumor size, may bias the real effects and conclusions they have made.19
Against this backdrop, we sought to examine the effects of RFA, SR, and LT in treating early- and very-early-stage HCC patients by using SEER database. We relied on the propensity-weighted and instrumental variable analysis (IVA) to account for measurable/unmeasurable confounders and competing-risk methodology to reduce the confounding effect of other-cause HCC patient mortality.
Materials and Methods
Patient Selection Criteria
We queried the SEER database for HCC patients with a histological diagnosis (International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3] code 8170) between 2004 and 2015. HCC Patients aged ≥18 years with tumor size, tumor grade, intrahepatic vascular invasion status, number of tumors, fibrosis score, and metastatic disease status who were not diagnosed at autopsy were included in the current study. From the above cohort, HCC patients with a single tumor measuring ≤50 mm who received RFA, SR, or LT were included for our comparison. We selected the following SEER codes for liver (C220): RFA: 16; SR: 20 to 25, 30, 36, 37, 50, 51, and 52; and LT: 61 and 66. Other types of ablation were excluded because RFA is the only ablative treatment with specific coding in the SEER database. Patients with incomplete survival data or a survival of <1 month were excluded from the study.
Outcome Measurement
The primary outcome was overall survival (OS), which is defined in SEER as time until death due to any cause. Disease-specific survival (DSS), which is defined as time until death due to HCC, was evaluated as a secondary outcome.
Statistical Analyses
Firstly, we examined the distribution of baseline characteristics between treatment groups using a two-sample t-test and a chi-square test (or Fisher exact test) to compare continuous and categorical variables, respectively. We presented continuous variables using means and corresponding standard deviations, while categorical variables were reported using frequencies and proportions.
Secondly, we used Cox proportional hazards and Fine-Gray competing risks regression models with propensity scores included as covariates to control for confounding to evaluate OS and DSS between treatments. Effect estimates are reported as cause-specific hazard ratios for Cox models or subdistribution hazard ratios (SHR) for Fine-Gray models, with 95% CIs. We estimated propensity scores with multinomial logistic regression, with treatment (RFA, LT, and SR) as the outcome and age, sex, tumor grade, tumor stage, chemotherapy received, tumor size, lymph nodes, AFP, fibrosis score, and prognostic covariates. Inverse probability of treatment weights (IPTWs) was calculated with the estimated propensity scores. To assess balance, standardized mean differences in covariate values were compared across treatment groups in an IPTWs sample.19 A standardized mean difference less than 0.1 has been suggested as a cutoff for adequate balance.20 If this was not achieved for a particular covariate, that covariate was then included in the Cox and Fine-Gray models in addition to the propensity scores. Covariates-adjusted survival curves and cumulative incidence estimates were generated with Kaplan–Meier methods with IPTWs.19 CIs for IPTWs-adjusted cumulative incidence estimates were obtained by bootstrapping. Finally, we then plotted treatment trends over time for early-stage HCC. Annual percent change (APC) in the delivery of SR, LT versus RFA was calculated using linear regression.
Thirdly, to account for selection bias between patients who received RFA, LT, and SR, we used an instrumental variable analysis (IVA) Rubin causal model21,22 to account for measured differences in baseline characteristics, as well as unmeasured confounders. We selected health service area (HSA), defined as one or more counties that are relatively self-contained with respect to the provision of routine hospital care, as our instrumental variable. The instrumental variable was constructed by first calculating the proportion of patients who received RFA or SR in each HSA. We computed the F-statistic to confirm its adequate correlation with the receipt of RFA or SR, while the second IVA assumption was assumed to be met, as the absence of correlation between the instrument and the outcome of interest other than through the exposure of interest cannot be formally tested.21 We then subsequently used instrumental variable (IV)-adjusted Cox model to evaluate treatments on OS and DSS. Additionally, we assessed whether the effect of treatments varied according to available baseline clinical characteristics by using a locally weighted regression method. All analyses were completed with R version 3.3.2, at a 2-tailed level of significance of 0.05.
Results
Unweighted and IPTW Cohort Characteristics
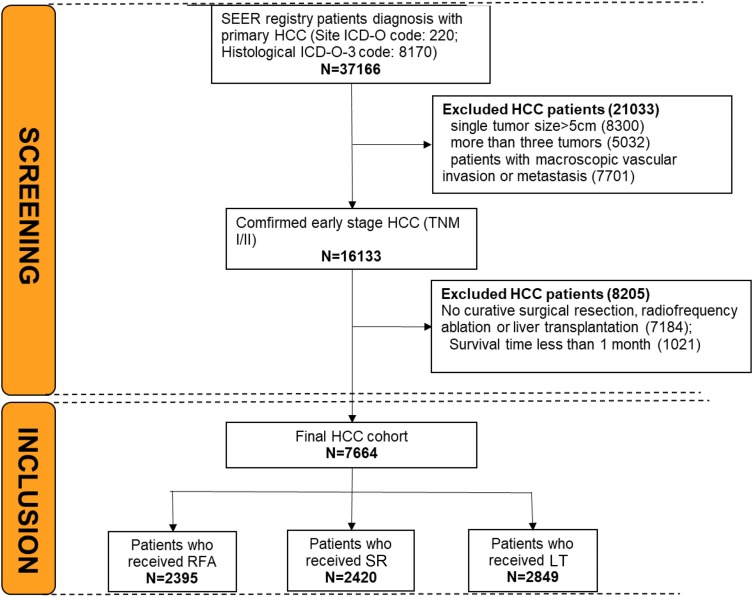
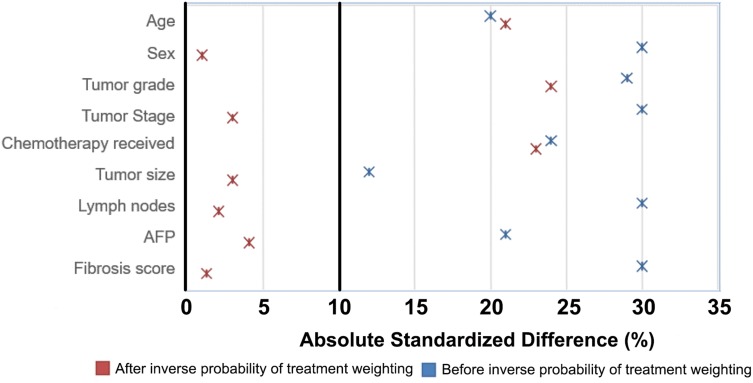
Of the 37,166 patients with a diagnosis of HCC in the SEER database, a total of 7664 met the criteria and then included in our study (Figure 1). Baseline clinical characteristics and treatment information are presented in Table 1 for early-stage HCC. 2395 patients underwent RFA, 2420 underwent SR, and 2849 underwent LT (Figure 1). The survival time between RFA, SR, and LT were 33.3, 38.5, and 57.6 months, respectively. Standardized differences of unweighted comparisons (Figure 2) showed that all treatment groups differed significantly with respect to several clinical, demographic, and tumor characteristics for three treatments. Specifically, after IPTW, all characteristics showed balance in treatments, except for tumor grade, chemotherapy received (Figure 2), which differed significantly between treatments and then they were included in the subsequent multivariate Cox models for further adjustment. Tumors were most often moderately differentiated. RFA (69%) and SR (70%) were equally distributed in patients with tumor stage I/II; and RFA was the most frequent treatment modality for patients with tumors measuring 21–30 mm.
Figure 1.
The study flowchart.
Abbreviations: RFA, radiofrequency ablation; SR, surgical resection; LT, liver transplantation.
Table 1.
Baseline Characteristics for Patients Who Received Radiofrequency Ablation, Surgical Resection, or Transplantation for Early and Very Early Stage Hepatocellular Carcinoma in the Unweighted and Weighted Study Populations by Stabilized IPTWs
| Characteristics | Unweighted Study Population | Weighted Study Population | ||||||
|---|---|---|---|---|---|---|---|---|
| RFA | SR | LT | P-Value* | RFA | SR | LT | P-Value | |
| No. of patients | 2395 | 2420 | 2849 | – | – | – | – | |
| Age | 64.2 ± 10.2 | 62.9 ± 11.1 | 57.2 ± 8.2 | <0.001 | 59.5 ± 10.0 | 60.8 ± 9.2 | 58.9 ± 7.7 | 0.0014 |
| Sex | <0.001 | 0.7017 | ||||||
| Female | 1775 (74.1%) | 1714 (70.8%) | 2216 (77.8%) | 74.2 | 76 | 75.9 | ||
| Male | 620 (25.9%) | 706 (29.2%) | 633 (22.2%) | 25.8 | 24 | 24.1 | ||
| Tumor grade | <0.001 | 0.024 | ||||||
| Well differentiated | 661 (43.3%) | 555 (26.0%) | 732 (35.8%) | 31.6 | 34.5 | 29.8 | ||
| Moderately differentiated | 715 (46.9%) | 1175 (55.0%) | 1097 (53.6%) | 58.3 | 51.2 | 55.6 | ||
| Poorly differentiated | 150 (9.8%) | 405 (19.0%) | 217 (10.6%) | 10.1 | 14.2 | 14.6 | ||
| AJCC. Tumor Stage | <0.001 | 0.8738 | ||||||
| I | 1537 (69.5%) | 1636 (70.9%) | 1436 (52.0%) | 59.6 | 60.8 | 57.9 | ||
| II | 653 (29.5%) | 659 (28.5%) | 1306 (47.3%) | 40 | 38.7 | 41.8 | ||
| III | 22 (1.0%) | 14 (0.6%) | 18 (0.7%) | 0.4 | 0.4 | 0.3 | ||
| Chemotherapy received | <0.001 | 0.038 | ||||||
| No | 1846 (77.1%) | 2167 (89.5%) | 1618 (56.8%) | 75.8 | 74 | 72.3 | ||
| Yes | 549 (22.9%) | 253 (10.5%) | 1231 (43.2%) | 24.2 | 26 | 27.7 | ||
| Tumor size | <0.001 | 0.6256 | ||||||
| <20 mm | 475 (21.6%) | 426 (18.4%) | 1087 (39.1%) | 28.4 | 29 | 25.7 | ||
| 21–30 mm | 875 (39.8%) | 687 (29.7%) | 907 (32.6%) | 36.2 | 32.3 | 36.9 | ||
| 31–50 mm | 547 (24.9%) | 859 (37.2%) | 492 (17.7%) | 23.6 | 26 | 24.9 | ||
| 31–35 mm | 301 (13.7%) | 339 (14.7%) | 293 (10.5%) | 11.8 | 12.6 | 12.5 | ||
| Lymph nodes | 0.286 | 0.9429 | ||||||
| No regional lymph node involvement | 2268 (99.0%) | 2316 (99.4%) | 2776 (99.4%) | 99.6 | 99.6 | 99.7 | ||
| Regional lymph nodes | 22 (1.0%) | 14 (0.6%) | 18 (0.6%) | 0.4 | 0.4 | 0.3 | ||
| AFP | 0.002 | 0.1217 | ||||||
| Negative/normal | 634 (33.1%) | 681 (37.9%) | 842 (38.9%) | 38.7 | 39.1 | 33.3 | ||
| Borderline/undetermined | 6 (0.3%) | 6 (0.3%) | 7 (0.3%) | 61.3 | 0.3 | 0.3 | ||
| Positive/elevated | 1277 (66.6%) | 1111 (61.8%) | 1316 (60.8%) | 60.6 | 66.5 | |||
| Fibrosis score | <0.001 | 0.2324 | ||||||
| F0 | 150 (19.1%) | 401 (45.5%) | 136 (10.8%) | 22.3 | 26.9 | 20.7 | ||
| F1 | 637 (80.9%) | 480 (54.5%) | 1125 (89.2%) | 77.7 | 73.1 | 79.3 | ||
| Cancer-specific survival status | <0.001 | |||||||
| Alive | 1102 (46.0%) | 1375 (56.8%) | 2301 (80.8%) | |||||
| Cancer-specific death | 818 (34.2%) | 583 (24.1%) | 333 (11.7%) | |||||
| Other-cause death | 475 (19.8%) | 462 (19.1%) | 215 (7.5%) | |||||
| Survival time | 33.3 ± 27.9 | 38.5 ± 32.0 | 57.6 ± 39.7 | <0.001 | ||||
Note: P-value* Calculated before inverse of probability treatment-weighted.
Abbreviations: AFP, alpha-fetoprotein; RFA, radiofrequency ablation; SR, surgical resection; LT, liver transplantation; IPTW, inverse of probability treatment-weighted.
Figure 2.
Effect of inverse probability of treatment weighting adjustment on the baseline characteristics distribution of patients who received RFA versus SR and LT for early-stage hepatocellular carcinoma.
Abbreviations: RFA, radiofrequency ablation; SR, surgical resection; LT, liver transplantation.
Survival Outcomes
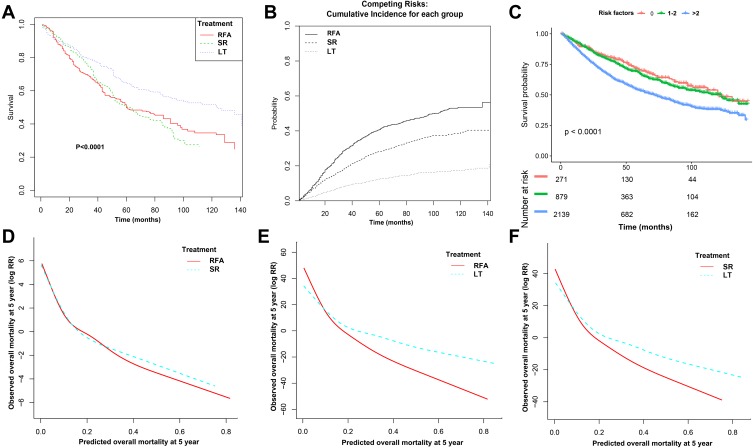
Median follow-up for the whole cohort was 55 months for OS and 43 months for DSS. Median follow-up for the RFA, SR, and LT groups was 45, 53, and 59 months, respectively, for OS and 41, 49, and 55 months for DSS. IPTW-adjusted Kaplan–Meier curves showed that treatment groups differed significantly in Figure 3A (P<0.001). IPTW-adjusted incidence rates and cumulative incidence rate estimates are shown in Figure 3B and Supplementary Table 1. Unadjusted, IPTW and propensity-score-adjusted Cox regression or competing risks regression models are shown in Table 2. Adjusted 3-year HCC-specific cumulative incidence rate were: RFA, 29.8% (CI, 27.8%, 31.9%); SR, 19.4% (CI, 17.7%, 21.2%); and LT, 8.7% (CI, 7.6%, 9.9%). In IPTW or propensity-score-adjusted models, patients who received LT versus RFA had longer 5-year OS (66% [CI, 59% to 74%] vs 52% [95% CI, 45% to 60%]) and lower mortality rates (adjusted HR, 0.6 [CI, 0.6 to 0.7], P<0.001) (supplementary tables 1 and Table 2). Similarly, in the disease-specific regression models and competing risk analyses, SHR for DSS also favored LT compared with RFA (HR, 0.6, CI: 0.6, 0.7, P<0.001) and RFA had higher 5-year disease-specific cumulative incidence (28.2% [CI, 26.0%, 30.3%]) than LT (12.5% [CI, 11.1%, 14.0%]). HR for OS did not favor SR vs RFA (HR, 0.9, CI: 0.8, 1.1) in the IPTW or propensity-score adjusted cohorts, although the confidence bounds reflect uncertainty of the estimates, and the differences in absolute survival were not large: 53% (CI, 46%, 62%) for RFA versus 52% (CI, 45%, 60%) for SR. SHR for DSS did favor SR vs RFA (SHR, 0.8, CI, 0.7. 0.9, P=0.002), and RFA had significant higher 5-year disease-specific cumulative incidence (40.7%, CI, 38.3%, 43.1%) than SR (28.2%, CI, 26.0%, 30.3%). Similarly, in terms of comparison for LT vs SR, lower overall mortality (HR, 0.6, CI: 0.6, 0.7, P<0.001) and HCC-specific mortality (SHR, 0.6, CI: 0.6, 0.7, P<0.001) favored LT and the differences in absolute survival were large: 52% (CI, 45%, 60%) for SR versus LT 66% (CI: 59%, 74%). Additionally, our sensitive analysis showed similar results in favoring SR vs RFA, LT vs RFA, and LT vs SR after IVA-adjusted analysis. The F-statistics for the HSA of SR vs RFA (137.27, P< 0.0001), LT vs RFA (15.16, P=0.002), and LT vs SR (70.24, P<0.0001) were greater than 10, which indicated that the first IVA assumption was met for our analyses (Supplementary Table 2).
Figure 3.
Inverse probability treatment weighting-adjusted Kaplan–Meier analysis of treatments (A) Cancer risk groups (C) for overall survival, cumulative incidence rate for disease-specific survival (B), and locally weighted curves depicting the predicted risk of overall mortality after (D) RFA and SR or (E) RFA and LT or (F) SR and LT.
Abbreviations: RFA, radiofrequency ablation; SR, surgical resection; LT, liver transplantation.
Table 2.
Associations of the Type of Treatments Oncologic Outcomes for Hepatocellular Carcinoma After Further Adjustment for Pathologic Features
| Model and Parameter | Cause-Specific Hazard Ratio (95% CI) | P-Value |
|---|---|---|
| HCC overall survival | ||
| Unadjusted cohort | ||
| SR vs RFA | 1.0 (0.8, 1.2) | 0.478 |
| LT vs RFA | 0.7 (0.6, 0.8) | <0.001 |
| vs SR | 0.3 (0.2, 0.3) | <0.001 |
| IPTW cohort | ||
| SR vs RFA | 1.0 (0.9, 1.1) | 0.478 |
| LT vs RFA | 0.6 (0.6, 0.7) | <0.001 |
| vs SR | 0.7 (0.6, 0.7) | <0.001 |
| IPTW and adjusted for some features (including age, fibrosis score, tumor grade and marital status) | ||
| SR vs RFA | 0.9 (0.8, 1.1) | 0.295 |
| LT vs RFA | 0.6 (0.6, 0.7) | <0.001 |
| vs SR | 0.6 (0.6, 0.7) | <0.001 |
| Cancer-specific survival | ||
| Unadjusted cohort | ||
| SR vs RFA | 0.9 (0.5, 0.9) | 0.047 |
| LT vs RFA | 0.5 (0.3, 0.9) | <0.011 |
| vs SR | 0.4 (0.3, 0.5) | <0.001 |
| IPTW cohort | ||
| SR vs RFA | 0.7 (0.5, 0.9) | 0.013 |
| LT vs RFA | 0.7 (0.6, 0.8) | <0.001 |
| vs SR | 0.3 (0.2, 0.5) | <0.001 |
| IPTW and adjusted for some features (including age, fibrosis score, tumor grade and marital status) | ||
| SR vs RFA | 0.8 (0.7, 1.0) | 0.002 |
| LT vs RFA | 0.6 (0.6, 0.7) | <0.001 |
| vs SR | 0.6 (0.6, 0.7) | <0.001 |
Abbreviations: IPTW, inverse of probability treatment-weighted; RFA, radiofrequency ablation; SR, surgical resection; LT, liver transplantation; HCC, hepatocellular carcinoma.
Cancer Risk Groups Associated with OS and Subgroup Analyses
Independent prognostic factors associated with OS in univariate and multivariate analysis are shown in Supplementary Table 3. These independent risk factors, including higher tumor grade, larger tumor size, higher fibrosis score, and higher levels of AFP, are significantly associated with poorer OS in early-stage HCC. Hence, we then stratified HCC patients into different risk groups according to the number of prognostic factors. The IPTW-adjusted Kaplan–Meier analysis of cancer risk groups differed significantly for OS in HCC (Figure 3C). Subgroup analysis according to tumor size and cancer risk factors for early- and very-early-stage hepatocellular carcinoma in overall and cancer-specific survival outcomes after reweighting by stabilized IPTWs are shown in Table 3.
Table 3.
Subgroup Analysis According to Tumor Size and Cancer Risk Factors for Early- and Very-early-stage Hepatocellular Carcinoma in Overall and Cancer-specific Survival Outcomes, After reweighting by Stabilized IPTWs
| Parameter | No. of Patients | Overall Survival | Cancer-Specific Survival* | ||||
|---|---|---|---|---|---|---|---|
| SR vs RFA | LT vs RFA | LT vs SR | SR vs RFA | LT vs RFA | LT vs SR |
||
| Tumor size | |||||||
| <20 mm | 104 | 0.7 (0.6, 0.8); P<0.0002 | 0.3 (0.2, 0.4); P<0.0001 | 0.8 (0.6, 1.2); P=0.3650 | 0.6 (0.5, 0.7); P<0.0001 | 0.28 (0.25,0.32); P<0.0001 | 0.44 (0.38,0.52); P<0.0001 |
| 21–30 mm | 112 | 1.1 (0.1, 9.5); P=0.929 | 0.5 (0.1, 3.7); P=0.476 | 0.9 (0.6, 1.2); P=0.4157 | 0.70 (0.57,0.86); P=0.0011 | 0.35 (0.28,0.44); P<0.0001 |
0.50 (0.39,0.65); P<0.0001 |
| 31–50 mm | 62 | 0.8 (0.7, 0.9); P=0.0089 | 0.1 (0.0, 0.2); P<0.001 | 0.5 (0.3, 0.6); P<0.0001 | 0.50(0.41, 0.6 1); P<0.0001 | 0.32 (0.24, 0.41); P<0.0001 | 0.62 (0.47, 0.81); P=0.0005 |
| 31–35 mm | 46 | 0.2 (0.0, 2.1); P=0.183 | 0.1 (0.0, 1.2); P=0.069 | 0.9 (0.6, 1.2); P=0.4157 | 0.56 (0.41, 0.76); P=0.0003 | 0.37 (0.26, 0.54); P<0.0001 | 0.66 (0.45, 0.96); P=0.0339 |
| P for interaction | – | 0.0230 | 0.5596 | ||||
| No. of prognostic risk factors | |||||||
| 0 | 271 | 1.2 (0.8, 1.9); P=0.4051 | 0.5 (0.1, 3.6); P=0.4850 | 0.2 (0.0, 0.9); P=0.0379 | 1.1(0.3, 4.2); P=0.8597 | 0.61 (0.17, 2.12); P=0.4425 | 0.54 (0.14, 2.03); P=0.3678 |
| 1–2 | 879 | 0.6 (0.4, 0.9); P=0.023 | 0.3 (0.2, 0.4); P<0.001 | 0.4 (0.2, 0.7); P=0.002 | 0.87 (0.50, 1.53); P=0.6514 | 0.43 (0.19, 0.94); P=0.0364 | 0.49 (0.21, 1.11); P=0.0903 |
| >2 | 2139 | 0.7 (0.6, 0.9); P=0.010 | 0.2 (0.0, 0.9); P=0.0379 | 0.4 (0.3, 0.5); P<0.001 | 1.06(0.87, 1.31); P=0.5269 | 0.72 (0.57, 0.92); P=0.0095 | 0.55 (0.34, 0.89); P=0.0166 |
| P for interaction | 0.8132 | 0.0247 | |||||
Notes: Overall survival was evaluated by hazard ratio (95% CI); cancer-specific survival* was analyzed by competing risks regression model of treating other-cause mortality as a competing risk, which was evaluated by subdistribution hazard ratio (95% CI).
Abbreviations: RFA, radiofrequency ablation; SR, surgical resection; LT, transplantation; HCC, hepatocellular carcinoma; IPTW, inverse of probability treatment-weighted.
Similarly, after stratifying patients into four groups according to tumor size, we found that there were significant effects in OS favoring SR vs RFA, LT vs RFA, and LT vs SR with tumor size measuring <20 mm and up to 50 mm (all HR<1); except for tumor size measuring 21 mm to 30 mm group, SR conferred more mortality risk than RFA (HR, 1.1, CI: 0.1, 9.5). However, for DSS outcome, all comparisons showed significant effects in favoring SR vs RFA, LT vs RFA, and LT vs SR with tumor size measuring <20 mm and up to 50 mm (all SHR <1), which indicated that LT was better than SR and SR was better than RFA regardless of tumor size.
In terms of cancer risk groups, we surprisingly found that SR was inferior to RFA (HR: 1.2, CI: 0.8, 1.9) for patients without any significant risk factors (tumor grade 0, tumor size <20 mm, fibrosis score/F0 and negative levels of AFP), and the similar results can be seen in DSS outcome (SHR, 1.1, CI: 0.3, 4.2), although no statistical significance can be reached (Table 3).
Finally, these independent risk factors were then used to predict 5-year overall mortality risk for the entire cohort and plotted against observed survival (Figure 3D–F). The locally weighted curves showed that there was no overall mortality-free survival difference between RFA and SR, regardless of the predicted risk of overall mortality at 5 years (P interaction= 0.7; Figure 3D). The lines for RFA and SR cross at 30%, indicating patients with a predicted overall mortality >30% will not benefit from RFA. In addition, the lines for RFA and LT, SR, and LT cross both at 15%, indicating patients with a predicted overall mortality >15% will not benefit from SR and RFA. The interaction between predicted overall mortality and RFA vs LT, SR vs LT was significant (P=0.01 and 0.03, respectively).
Discussion
The optimal treatment strategy for patients with early and very early stage HCC is a subject of continuous development. Specifically, liver transplantation is the best treatment option with curative treatment for patients with HCC,23 which is also concluded from our study. Unfortunately, because of a shortage of available liver donors, SR and RFA are still more appropriate treatments for a larger scale. Even though SR is regarded as an effective treatment with HCC patients, the morbidity of liver resection added to the risks of SR in the liver has been a limiting factor.18,24–27 However, RFA has been widely used, especially in patients with significant underlying parenchymal disease,28 and is regarded as the first-line therapy for patients with very early and early HCC (BCLC stages 0-A) who are not amenable to liver resection.29
Hence, in this IPTW-adjusted cohort study, we sought to evaluate the relative efficacy of RFA, LT, and SR as first-line therapy for patients with early HCC by using IPTW-adjusted or IVA models.19,22 Furthermore, we aimed to determine whether there are differences on survival between RFA, LT, and SR when used as first-line therapy for patients with HCC tumor sizes and cancer risk groups. Locally weighted curve analyses between treatments were also explored. The results from the current analysis confirm reports that SR is an effective option for patients with different HCC tumor size, except for measuring between 21 mm to 30 mm (comparable mortality risk was achieved with no statistical significance; HR=1.1, P=0.929), which is inconsistent with previous study.18 However, RFA may be an alternative treatment compared with SR with no statistical significance (HR=1.2, P=0.405) for HCC patients with no prognostic risk factors including lowest tumor grade, least tumor size, lowest fibrosis score (F0), and negative level of AFP. Specifically, those with a predicted 5-year overall mortality risk >30% seem to benefit the more for SR comparing RFA.
In line with previous studies, a recent Japanese study compared RFA with SR in patients with small, poorly differentiated HCC tumors: the maximum tumor size was 2 cm in the RFA group and while 2.5 cm in the SR group.12,30 RFA for these small tumors was found to be significantly inferior, demonstrating a cumulative 5-year survival rate of 32.7%, whereas SR yielded a survival rate of 67.5%; the recurrence-free survival rate also was found to be significantly lower in the RFA group.31 In addition, a Japanese nationwide survey of 12,968 patients with tumors measuring <3 cm found significantly lower odds of death and disease recurrence in patients who received SR compared with those who received RFA.32 Similarly, in a randomized controlled trial from China with 230 HCC patients, worse 5-year OS and recurrence-free survival as well as higher overall tumor recurrence were revealed in the RFA group.30 However, among patients with favorable tumor characteristics (lowest tumor grade, least tumor size and fibrosis score), RFA may be an alternative treatment compared with SR.
According to the guidelines,33,34 RFA is the appropriate treatment for single lesions <5 cm or up to 3 lesions <3 cm, with several limitations in performance, especially the vicinity to heat-sensitive organs. For favorable tumor or clinicopathological characteristics of HCC patients (with no risk factors), RFA may confer more survival benefits than SR. Patients presenting one or more risk factors should be considered for SR rather than RFA. In line with locally weighted curve analysis, although there were no survival differences between two treatments, the lines for RFA and SR cross at 30%, indicating patients with a predicted overall mortality >30% will not benefit from RFA. As previous studies report, SR has traditionally been recommended as the first-line treatment option for patients with small solitary HCC. The 5-year OS rate for small solitary HCC (<2 cm) or in BCLC very early stage ranges from 60% to 100%.30,35 For solitary small HCC, minute or small satellite tumors might be present near the primary tumor in at least 13% cases, which were not detected by imaging studies. In a randomized study, partial liver resection with a wide resection margin improved survival outcome and decreased recurrence rate for solitary HCC <2 cm.30,36 The advantage of anatomical subsegmentectomy in complete resection of tumor tissue and portal territory containing the tumor also contributes to the lower frequency of intrahepatic recurrence by SR. Compared with RFA, these might explain more survival benefits after SR in our study. For the cancer subgroup analysis and locally weighted curve results, patients involving more unfavorable prognostic factors represent higher tumor grade or fibrosis score; complete resection of tumor tissue and underlying aggressive factors may contribute to higher survival rates for SR than RFA. For patients with BCLC very-early-stage HCC, our result is different from the conclusion made by Hung et al.37 In Hung’s study, despite SR yielding a 5-year cumulative recurrence rate higher than the RFA group (74.8% vs. 54.8%), comparable OS and recurrence rates were obtained between SR and RFA.37 However, the results need further evaluation due to small numbers of patients (23 in the RFA group and 25 in the SR group) enrolled in the propensity scores matching model analysis. Similarly, Kutlu et al18 reported that although RFA is an appropriate option for patients with HCC tumors measuring <30 mm, its use for tumors even slightly larger than 30 mm is associated with inferior outcomes. However, potential treatment residual bias and unmeasurable confounders may affect the above results; we hence applied propensity-score-adjusted IPTW and IVA analysis to account for measurable/unmeasurable confounders and competing-risk methodology to reduce the confounding effect of other-cause HCC patient mortality. Therefore, in clinical practice, the feasibility of SR as initial treatment should be assessed before RFA. When SR is not feasible at initial assessment, RFA might be the treatment of choice, taking into account its low invasiveness and high effectiveness in local tumor control. However, SR should be re assessed for those patients who failed local tumor control by RFA. For patients who underwent SR after initial local failure by RFA, the OS was identical to that of patients who underwent SR initially.38
Our study is not devoid of limitations. Firstly, our study is limited by the retrospective nature of the entire cohort. In order to overcome this lack, we applied PS score analysis and IVA analysis to control residual treatment selection bias. Secondly, the SEER database does not provide the information about tumor location, which might be the cause of outcome difference between SR and RFA. In general, HCC lesions located more in the center of the liver parenchyma are easier to ablate since a surgical approach in these cases may cause a larger parenchymal defect than RFA with a higher risk for liver dysfunction and subsequently worse outcome. An additional limitation of our study was the lack of information regarding portal hypertension and the actual fibrosis score. Although there were information available regarding the severity of fibrosis, this was recorded as either none-moderate (score of 0–4) or severe-cirrhosis.15,39,40 To compensate for this limitation, we chose to analyze both OS and DSS when evaluating the treatments. The goal was to include the burden of the underlying disease and the general condition of the patient in our analysis. Evaluating the outcome of DSS only provided information regarding the outcomes because of the malignant process, thereby ignoring the underlying liver disease. Conversely, evaluating OS indirectly incorporates the impact of the underlying liver disease or severe fibrosis.15
In conclusion, relying on population-based cohort, in HCC patients with early and very early stage, LT would be the ideal treatment modality because the underlying disease process can be addressed, not only the tumor itself. Despite the exception points assigned to HCC patients, a shortage of donors continues to be the central issue. For this reason, RFA and SR can be applied as either primary management of HCC or as a bridging therapy for LT; also, our current study validated SR and RFA as reasonable bridging treatments. Our conclusion is based on data from the study showing that for HCC patients with small tumors, SR may confer more survival benefits than RFA for different tumor sizes measuring up to 5 cm and is an appropriate first-line treatment. Additionally, RFA has more survival benefits compared with SR for those patients with low tumor risk and good general health condition. However, those patients with a predicted 5-year overall mortality risk >30% seem to benefit more for SR than RFA.
Abbreviations
HCC, hepatocellular carcinoma; OS, overall survival; CI, confidence interval; HR, hazard risk; IPTW, inverse of probability treatment-weighted; RFA, radiofrequency ablation; SR, surgical resection; LT, liver transplantation; OS, overall survival; DSS, disease-specific survival; BCLC, Barcelona Clinic for Liver Cancer; IVA, instrumental variable analysis; APC, annual percent change.
Availability of Data and Material
All data generated or analyzed during this study are from SEER datasets.
Author Contributions
Zhao WJ, Zhu GQ, Wu YM, and Bai BL designed the study. Zhao WJ, Zhu GQ collected and downloaded data. Zhu GQ, Wu YM did the statistical analyses. Bai BL and Zhu GQ prepared figures. Zhao WJ, Zhu GQ, Wu YM, and Bai BL reviewed the results, interpreted data, and wrote the manuscript. All authors have made an intellectual contribution to the manuscript and approved the submission. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Benson AB 3rd, D’Angelica MI, Abbott DE, et al. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15(5):563–573. doi: 10.6004/jnccn.2017.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 4.Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30(1):3–16. doi: 10.1055/s-0030-1247128 [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 6.Akoad ME, Pomfret EA. Surgical resection and liver transplantation for hepatocellular carcinoma. Clin Liver Dis. 2015;19(2):381–399. doi: 10.1016/j.cld.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 7.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon’s perspective. Ann Surg. 2002;235(4):466–486. doi: 10.1097/00000658-200204000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hocquelet A, Balageas P, Laurent C, et al. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: a study of 281 Western patients. Int J Hyperthermia. 2015;31(7):749–757. doi: 10.3109/02656736.2015.1068382 [DOI] [PubMed] [Google Scholar]
- 9.Tschuor C, Croome KP, Sergeant G, et al. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion – an extension of the ALPPS approach. Eur J Surg Oncol. 2013;39(11):1230–1235. doi: 10.1016/j.ejso.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 10.Wang CH, Wey KC, Mo LR, Chang KK, Lin RC, Kuo JJ. Current trends and recent advances in diagnosis, therapy, and prevention of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2015;16(9):3595–3604. doi: 10.7314/APJCP.2015.16.9.3595 [DOI] [PubMed] [Google Scholar]
- 11.Gory I, Fink M, Bell S, et al. Radiofrequency ablation versus resection for the treatment of early stage hepatocellular carcinoma: a multicenter Australian study. Scand J Gastroenterol. 2015;50(5):567–576. doi: 10.3109/00365521.2014.953572 [DOI] [PubMed] [Google Scholar]
- 12.Orlacchio A, Chegai F, Del Giudice C, et al. Radiofrequency thermoablation of HCC larger than 3 cm and less than 5 cm proximal to the gallbladder without gallbladder isolation: a single center experience. Biomed Res Int. 2014;2014:896527. doi: 10.1155/2014/896527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thandassery RB, Goenka U, Goenka MK. Role of local ablative therapy for hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S104–S111. doi: 10.1016/j.jceh.2014.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iida H, Aihara T, Ikuta S, Yamanaka N. Comparative study of percutaneous radiofrequency ablation and hepatic resection for small, poorly differentiated hepatocellular carcinomas. Hepatol Res. 2014;44(10):E156–E162. doi: 10.1111/hepr.2014.44.issue-10 [DOI] [PubMed] [Google Scholar]
- 15.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. doi: 10.1097/01.sla.0000201480.65519.b8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogihara M, Wong LL, Machi J. Radiofrequency ablation versus surgical resection for single nodule hepatocellular carcinoma: long-term outcomes. HPB (Oxford). 2005;7(3):214–221. doi: 10.1080/13651820510028846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santambrogio R, Opocher E, Montorsi M. Laparoscopic radiofrequency ablation of hepatocellular carcinoma: a critical review from the surgeon’s perspective. J Ultrasound. 2008;11(1):1–7. doi: 10.1016/j.jus.2007.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutlu OC, Chan JA, Aloia TA, et al. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer. 2017;123(10):1817–1827. doi: 10.1002/cncr.v123.10 [DOI] [PubMed] [Google Scholar]
- 19.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 20.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. doi: 10.1016/S0895-4356(00)00321-8 [DOI] [PubMed] [Google Scholar]
- 21.Stel VS, Dekker FW, Zoccali C, Jager KJ. Instrumental variable analysis. Nephrol Dial Transplant. 2013;28(7):1694–1699. doi: 10.1093/ndt/gfs310 [DOI] [PubMed] [Google Scholar]
- 22.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 23.Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986–994.e983. doi: 10.1053/j.gastro.2012.05.052 [DOI] [PubMed] [Google Scholar]
- 24.Kang TW, Kim JM, Rhim H, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection–propensity score analyses of long-term outcomes. Radiology. 2015;275(3):908–919. doi: 10.1148/radiol.15141483 [DOI] [PubMed] [Google Scholar]
- 25.Uhlig J, Sellers CM, Stein SM, Kim HS. Radiofrequency ablation versus surgical resection of hepatocellular carcinoma: contemporary treatment trends and outcomes from the United States National Cancer Database. Eur Radiol. 2019;29(5):2679–2689. doi: 10.1007/s00330-018-5902-4 [DOI] [PubMed] [Google Scholar]
- 26.Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56(2):412–418. doi: 10.1016/j.jhep.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014;9(1):e84484. doi: 10.1371/journal.pone.0084484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livraghi T. Single HCC smaller than 2 cm: surgery or ablation: interventional oncologist’s perspective. J Hepatobiliary Pancreat Sci. 2010;17(4):425–429. doi: 10.1007/s00534-009-0244-x [DOI] [PubMed] [Google Scholar]
- 29.Munene G, Vauthey JN, Dixon E. Summary of the 2010 AHPBA/SSO/SSAT consensus conference on HCC. Int J Hepatol. 2011;2011:565060. doi: 10.4061/2011/565060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104(13):1775–1784. doi: 10.1002/bjs.10677 [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58(4):724–729. doi: 10.1016/j.jhep.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 32.Ni JY, Xu LF, Sun HL, Zhou JX, Chen YT, Luo JH. Percutaneous ablation therapy versus surgical resection in the treatment for early-stage hepatocellular carcinoma: a meta-analysis of 21,494 patients. J Cancer Res Clin Oncol. 2013;139(12):2021–2033. doi: 10.1007/s00432-013-1530-1 [DOI] [PubMed] [Google Scholar]
- 33.Covey AM. Hepatocellular carcinoma: updates to screening and diagnosis. J Natl Compr Canc Netw. 2018;16(5s):663–665. doi: 10.6004/jnccn.2018.0052 [DOI] [PubMed] [Google Scholar]
- 34.Kuo KL, Stenehjem D, Albright F, Ray S, Brixner D. Treatment patterns and outcomes in patients with hepatocellular carcinoma stratified by stage-guided treatment categories. J Natl Compr Canc Netw. 2015;13(8):987–994. doi: 10.6004/jnccn.2015.0119 [DOI] [PubMed] [Google Scholar]
- 35.Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245(1):36–43. doi: 10.1097/01.sla.0000231758.07868.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langenbach MC. RFA vs resection of HCC: exploring the past to improve the future. Eur Radiol. 2019;29(5):2677–2678. doi: 10.1007/s00330-019-6000-y [DOI] [PubMed] [Google Scholar]
- 37.Hung HH, Chiou YY, Hsia CY, et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol. 2011;9(1):79–86. doi: 10.1016/j.cgh.2010.08.018 [DOI] [PubMed] [Google Scholar]
- 38.Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51(4):1284–1290. doi: 10.1002/hep.23466 [DOI] [PubMed] [Google Scholar]
- 39.Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307. doi: 10.1016/j.jhep.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 40.Duan C, Liu M, Zhang Z, Ma K, Bie P. Radiofrequency ablation versus hepatic resection for the treatment of early-stage hepatocellular carcinoma meeting Milan criteria: a systematic review and meta-analysis. World J Surg Oncol. 2013;11(1):190. doi: 10.1186/1477-7819-11-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are from SEER datasets.