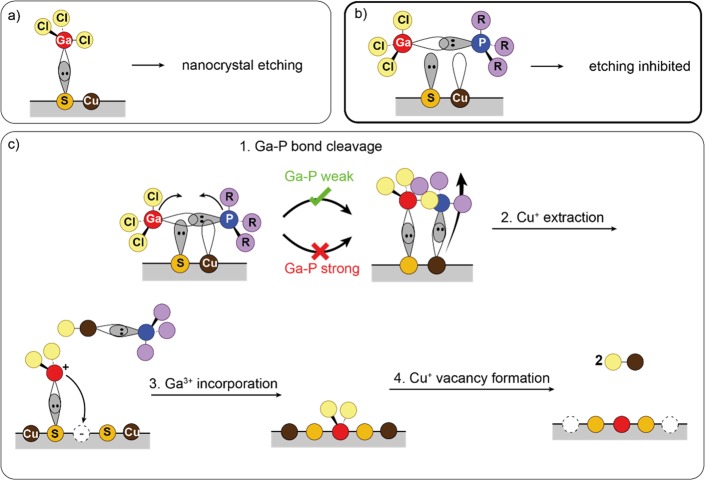
Figure 7.
Schematic depiction of the Cu+ for Ga3+ cation exchange reaction in Cu2–xS nanocrystals. (a) Free GaCl3 reacts as a Lewis acid, extracting S2–, leading to etching of the nanocrystals. (b) Extraction of S2– is inhibited when GaCl3 is complexed with a phosphine ligand. (c) Successful cation exchange involves four steps: (i) cleavage of the Ga–P bond at the Cu2–xS nanocrystal surface, at which point Ga–S and P–Cu bonds are made simultaneously; (ii) extraction of Cu+ by the phosphine; (iii) subsequent incorporation of Ga3+ into the nanocrystal; and (iv) reaction of remaining Cl– with Cu+, extracting two additional Cu+ ions, completing the exchange.