Abstract
Rationale:
Occasionally, tubulointerstitial lesions can be found in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). However, significantly isolated tubulointerstitial nephritis (TIN) with germinal centers is rare.
Patient concerns:
A 17-year-old Chinese Han patient showed rapidly progressive glomerulonephritis, anuria, and serum creatinine of 19.4 mg/dL.
Diagnosis:
He had positive ANCA targeting myeloperoxidase (55.0 RU/mL). The renal biopsy showed crescent formation in 100% of glomeruli. Of special note, the glomerular crescents were surrounded by granulomatous inflammation, extensive tubular destruction or disappearance, and massive interstitial infiltration. A diagnosis of AAV was thus made with the involved organ restricted to the kidney.
Interventions:
The patient underwent 7 rounds of plasmapheresis, 3 pulses of methylprednisolone therapy (500 mg per pulse), and oral prednisolone (50 mg/d). Rituximab (500 mg) was used after the plasma exchange treatment.
Outcomes:
ANCA was negative, while anti-modified C-reactive protein (anti-mCRP) antibodies remained positive. The patient was dependent on hemodialysis. We found anti-mCRP antibody in the serum of the patient, with the major epitope on amino acids 35 to 47 of mCRP.
Lessons:
We proposed that the anti-mCRP antibody might play an important role in this case of acute TIN in AAV.
Keywords: anti-mCRP antibody, antineutrophil cytoplasmic antibody-associated vasculitis, germinal center, rapidly progressive glomerulonephritis, tubulointerstitial nephritis
1. Introduction
Renal involvement is common in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), and patients often present with pauci-immune necrotizing crescentic glomerulonephritis.[1] Tubulointerstitial (TI) lesions can also be found in the kidneys in AAV, although their pathogenesis remains to be elucidated.[1–3] Modified C-reactive protein (mCRP) is a tissue and/or cell-based form of the acute phase protein and has been suggested to be a possible antigen in acute tubulointerstitial nephritis (ATIN). However, there is no report of an anti-mCRP antibody in patients with AAV. Here, we present a case of ATIN in AAV with positive serum anti-mCRP antibody, which might provide some insights into the pathogenesis of the disease.
2. Case presentation
A 17-year-old Chinese Han man was admitted with a 23-day history of edema, fatigue, and subsequent anuria. He had no fever. Ten days before admission, his serum creatinine was 19.4 mg/dL, and urinalysis revealed proteinuria 1+ and hematuria with 228 red blood cells/high-power field (HPF). He was positive for perinuclear ANCA (pANCA) by immunofluorescence. He received hemodialysis and renal biopsy and was referred to our hospital. He had suffered from hyperthyroidism for 7 years and had been prescribed thiamazole and propylthiouracil (PTU) for 3 years. Moreover, he had a 5-year history of allergic rhinitis and allergic asthma. He had no family history of hypertension, glomerulonephritis or end-stage renal disease, and he had no drug addiction.
On admission, the physical examination revealed a blood pressure of 128/73 mm Hg, temperature of 36.5°C, heart rate of 78/min, and respiratory rate of 20/min. The patient was anemic, although further systemic clinical examination was unremarkable.
The laboratory data revealed serum creatinine of 12.3 mg/dL and interleukin-6 of 66.0 pg/mL (normal range: 0–0.64 pg/mL). The patient's urinalysis revealed proteinuria 1+ and dysmorphic red blood cells >100/HPF; he was positive for pANCA by immunofluorescence, and anti-MPO antibody was shown to be positive at 55.0 RU/mL by enzyme-linked immunosorbent assay. Anti-glomerular basement membrane antibody and antinuclear antibody were negative. The ophthalmological examination excluded tubulointerstitial nephritis with uveitis (TINU), and positron emission tomography-computed tomography scan indicated no tumor or infection.
The renal histology showed diffuse destruction of glomerular structure with crescents and severely ruptured Bowman capsules (Fig. 1), which were surrounded by granulomatous inflammation, massive destruction or disappearance of tubules, and extensive interstitial infiltration of lymphocytes, monocytes, eosinophils, and plasma cells, with focal lymphocyte aggregation into “germinal centers” (Fig. 1). Immunofluorescence microscopy showed little, if any, deposition of immunoglobulin or complement in the glomeruli and tubulointerstitium. Immunohistochemical staining (Fig. 1) showed that the interstitial infiltrated cells were positive for CD20 (++), CD3 (+), CD138 (++), and Bcl2 (+), and negative for CD23, CyclinD1 and CD30.
Figure 1.
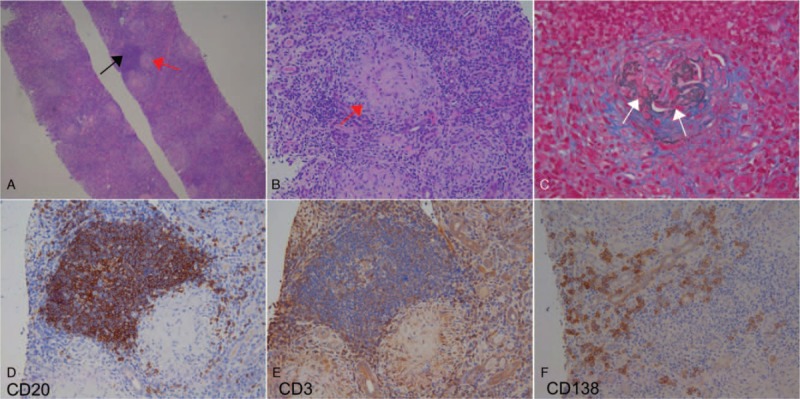
The pathological findings of renal biopsy. (A) The renal parenchyma was diffusely destroyed, all glomeruli exhibited crescents, and Bowman capsules were disrupted and surrounded by a granuloma (red arrow), along with tubular atrophy and disappearance, massive interstitial infiltration of inflammatory cells, and the “germinal center” (black arrow) formation in the interstitium (HE ×40). (B) The granuloma (red arrow) showed the destruction of the glomerular structure and the disrupted Bowman capsule (HE ×200). (C) Disruption of the glomerular basement membrane (white arrows) surrounded by a granuloma (PASM + Masson ×200). Of one “germinal center” in the interstitium, CD20+ B cells (D) were clustered in the middle area and surrounded by CD3+ T cells (E). Scattered CD138+ plasma cells (F) were observed in the surrounding parenchyma (D–F, ×200).
Because of the severe TI injury observed in his kidney specimens, we detected anti-mCRP antibodies. The patient was strongly positive (112%) for serum anti-mCRP antibodies. As illustrated in Figure 2, the anti-mCRP antibody titer was 1:800, the subclasses of immunoglobulin G (IgG) were IgG2 and IgG3, and the major epitope was amino acids (a.a.) 35 to 47. Eight months later, the titer was still 1:800, while the main IgG subclass was IgG1.
Figure 2.
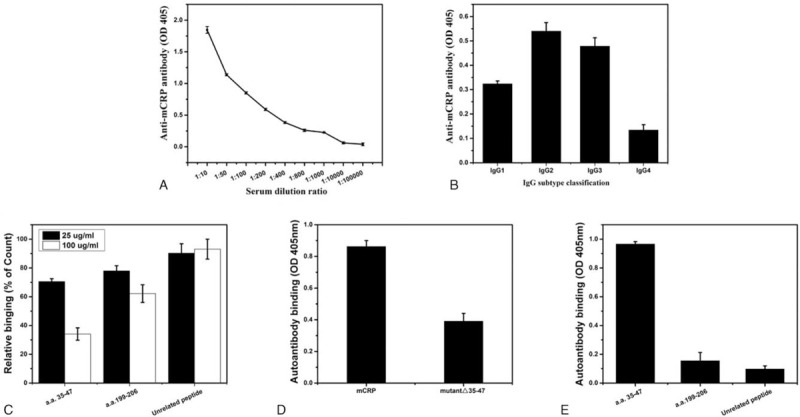
The anti-mCRP antibodies in the patient. (A) Titer, 1:800. (B) IgG subclass, IgG2, and IgG3. (C–E) Epitope, a.a. 35 to 47. (C) The serum of the patient was mixed with the indicated peptides and added to immobilized mCRP; a.a. 35 to 47 was the only peptide that significantly reduced the binding between mCRP and its antibodies. (D) mCRP and mutant mCRP lacking a.a. 35 to 47 (mutant Δ35–47) were expressed in Escherichia coli and purified, and their binding to immobilized anti-mCRP antibodies was examined. mCRP showed strong binding, whereas the binding capacity was lost upon deletion of a.a. 35 to 47. (E) Synthesized CRP peptides were immobilized and tested for binding to antibodies from this patient. Apparent binding to a.a. 35–47 was observed. a.a. = amini acids, IgG = immunoglobulin G, mCRP = modified C-reactive protein.
The patient received 7 rounds of plasmapheresis, 3 pulses of methylprednisolone therapy (500 mg per pulse), and oral prednisolone (50 mg/d). Rituximab (500 mg) was used after the plasma exchange treatment. ANCA was negative, while anti-mCRP antibodies remained positive. The patient was dependent on hemodialysis.
3. Discussion
This patient presented with rapidly progressive glomerulonephritis, positive serum ANCA and pauci-immune crescentic glomerulonephritis with severe interstitial nephritis. Thus, a diagnosis of AAV was highly suspected. However, several other diseases should be excluded.
First, PTU-induced ANCA-positive vasculitis must be highly suspected. Increasing evidence has demonstrated that PTU could induce ANCA-positive vasculitis, and the withdrawal of the drug was closely related to the improvement of active vasculitis and renal function.[4] However, this patient stopped taking PTU 4 years ago. Second, TINU syndrome also need to be considered. However, his ophthalmological examination was normal, and severe glomerular crescent formation was not consistent with the characteristics of TINU.
By excluding other possible diagnoses, a diagnosis of AAV was made with its target organ restricted to the kidney.
Renal involvement of AAV is predominant in glomerular lesions, especially pauci-immune necrotizing crescentic glomerulonephritis. The TI lesions are thought to be secondary damage to the glomeruli.[1–3] However, the current patient showed severe TI lesions with germinal centers and Bowman capsule rupture, which could not be explained simply by AAV.
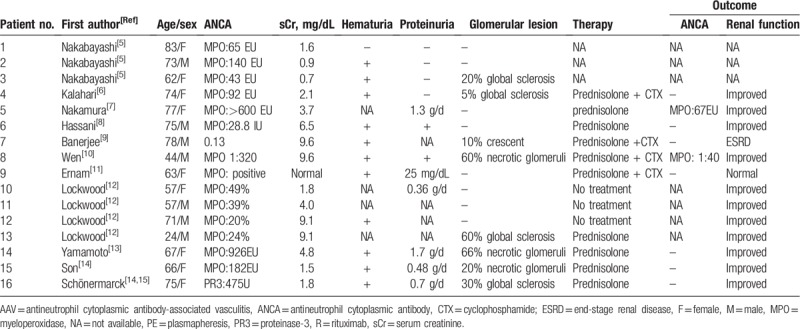
To our knowledge, there are 16 reported cases of TIN with positive ANCA (Table 1).[5–15] In some cases, renal function improved with decreased ANCA levels after immunosuppressive therapy. The mechanism of isolated TIN in AAV remains to be clarified. Sato et al[16] and Nakabayashi et al[5] proposed that peritubular capillary injury was the pathogenesis of the TI changes in AAV, and the latter suggested that early loss of CD34 antigenicity in the peritubular capillary played an important role. Kasahara et al[6] proposed that low-affinity MPO-ANCA may recognize some antigens specific to the TI area. Nakamura et al[7] and Hassani et al[8] proposed that endothelial tubular cell injury may be induced by the adhesion of leukocytes that express MPO and proteinase 3 on the cell surface. Banerjee et al[9] and Wen et al[10] proposed that activated neutrophils in the renal interstitium could contribute to a direct cell-mediated injury to the interstitium.
Table 1.
Summary of the reported case with tubulointerstitial nephritis with AAV.
mCRP is a tissue and/or cell-based form of the acute phase protein. Our previous study found that mCRP might be a target autoantigen in TINU syndrome,[17] which is characterized by TIN with bilateral sudden-onset anterior uveitis. The circulating level of anti-mCRP autoantibodies in patients with lupus nephritis (LN) was closely associated with the score of their interstitial lesions.[18] Recently, Li et al[19] showed that a.a. 35 to 47, a sequence exposed only in mCRP, constitutes the major epitope recognized by anti-CRP autoantibodies in patients with LN and indicates that mCRP binds complement factor H and enhances its cofactor activity via a.a. 35 to 47, whereas autoantibodies against this epitope inhibit these actions and adversely affect LN. The anti-mCRP autoantibody titer in this patient was high, and its IgG3 subclass is pathogenic. Furthermore, its epitope is a.a. 35 to 47. Thus, we suggest that anti-mCRP antibodies might play an important role in the patient's interstitial lesions.
More interestingly, there were many ectopic germinal centers and nonlymphoid collections of mature B lymphocytes in the renal interstitium of this patient, suggesting that severe renal interstitial inflammation may be associated with his autoimmune disease. The patient also presented with elevated interleukin (IL)-6. Recently, Espeli et al[20] showed that in LN, the kidneys are a major source of autoantibody-producing plasma cells. The inflammatory cytokine IL-6 supports long-lived plasma cell survival in vitro and is increased in the serum of patients with lupus. Arkatkar et al[21] demonstrated that B cell-derived IL-6 is critical for spontaneous germinal center formation. Ectopic germinal center formation may contribute to the progression of lupus interstitial nephritis by selecting for cells that locally secrete pathogenic antibodies in the tubulointerstitium.[22] It is unclear whether a similar process occurred in the current case, but further study is needed.
In conclusion, we reported a case of severe TI lesions with many germinal centers and rupture of all Bowman capsules in AAV, which may result from anti-mCRP antibodies.
4. Statement
The study was performed in compliance with the Declaration of Helsinki and approved by the Ethics Committee of Peking University First Hospital. Informed written consent was obtained from the patient for publication of this case report and accompanying images.
Author contributions
Methodology: Zi-Shan Lin, Xiao-Ling Liu.
Supervision: Zi-Shan Lin.
Validation: Zi-Shan Lin.
Visualization: Zi-Shan Lin.
Writing – original draft: Zi-Shan Lin.
Writing – review and editing: Zhao Cui, Su-Xia Wang, Feng Yu, Fu-De Zhou, Ming-Hui Zhao.
Footnotes
Abbreviations: AAV = antineutrophil cytoplasmic antibody-associated vasculitis, ANCA = antineutrophil cytoplasmic antibody, HPF = high-power field, LN = lupus nephritis, mCRP = anti-modified C-reactive protein, MPO = myeloperoxidase, pANCA = perinuclear ANCA, PTU = propylthiouracil, TI = tubulointerstitial, TIN = tubulointerstitial nephritis, TINU = tubulo-interstitial nephritis and uveitis.
How to cite this article: Lin ZS, Liu XL, Cui Z, Wang SX, Yu F, Zhou FD, Zhao MH. Acute tubulointerstitial nephritis with germinal centers in antineutrophil cytoplasmic antibody-associated vasculitis: a case report and literature review. Medicine. 2019;98:48(e18178).
The authors have no conflicts of interest to disclose.
References
- [1].Zonozi R, Niles JL, Cortazar FB. Renal involvement in antineutrophil cytoplasmic antibody-associated vasculitis. Rheum Dis Clin North Am 2018;44:525–43. [DOI] [PubMed] [Google Scholar]
- [2].Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010;21:1628–36. [DOI] [PubMed] [Google Scholar]
- [3].Berden AE, Jones RB, Erasmus DD, et al. Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis after rituximab therapy. J Am Soc Nephrol 2012;23:313–21. [DOI] [PubMed] [Google Scholar]
- [4].Gao Y, Chen M, Ye H, et al. Follow-up of avidity and titre of anti-myeloperoxidase antibodies in sera from patients with propylthiouracil-induced vasculitis. Clin Endocrinol 2007;66:543–7. [DOI] [PubMed] [Google Scholar]
- [5].Nakabayashi K, Sumiishi A, Sano K, et al. Tubulointerstitial nephritis without glomerular lesions in three patients with myeloperoxidase-ANCA-associated vasculitis. Clin Exp Nephrol 2009;13:605–13. [DOI] [PubMed] [Google Scholar]
- [6].Kasahara H, Hiroyuki N, Shinohara M, et al. AP-VAS 2012 case report: an atypical case of microscopic polyangiitis presenting with acute tubulointerstitial nephritis without glomerular change. CEN Case Rep 2014;3:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakamura N, Yaegaki M, Sugawara T, et al. Acute tubulointerstitial nephritis with antineutrophil cytoplasmic antibody. Hong Kong J Nephrol 2006;8:33–5. [Google Scholar]
- [8].Hassani K, Hamzi AM, Hassani M, et al. Acute tubulo-interstitial nephritis with positive anti-neutrophil cytoplasmic antibodies. Arab J Nephrol Transplant 2013;6:177–9. [PubMed] [Google Scholar]
- [9].Banerjee A, McKane W, Thiru S, et al. Wegener's granulomatosis presenting as acute suppurative interstitial nephritis. J Clin Pathol 2001;54:787–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wen YK, Chen ML. Transformation from tubulointerstitial nephritis to crescentic glomerulonephritis: an unusual presentation of ANCA-associated renal vasculitis. Renal Fail 2006;28:189–91. [DOI] [PubMed] [Google Scholar]
- [11].Ernam D, Atikcan S, Yilmaz A, et al. An unusual renal presentation of Wegener's granulomatosis. Tuberk Toraks 2003;51:193–6. [PubMed] [Google Scholar]
- [12].Lockwood CM. Antineutrophil cytoplasmic autoantibodies: the nephrologist's perspective. Am J Kidney Dis 1991;18:171–4. [DOI] [PubMed] [Google Scholar]
- [13].Yamamoto T, Yoshihara S, Suzuki H, et al. MPO-ANCA-positive crescentic necrotizing glomerulonephritis and tubulointerstitial nephritis with renal eosinophilic infiltration and peripheral blood eosinophilia. Am J Kidney Dis 1998;31:1032–7. [DOI] [PubMed] [Google Scholar]
- [14].Son D, Kanda H, Yamaguchi A, et al. Myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis with diffuse tubulointerstitial nephritis. J Nephrol 2009;22:417–20. [PubMed] [Google Scholar]
- [15].Schonermarck U, Schirren CA, Mistry-Burchardi N, et al. Interstitial nephritis and high titers of PR3-ANCA: an unusual manifestation of ANCA-associated disease. Clin Nephrol 2005;64:383–6. [DOI] [PubMed] [Google Scholar]
- [16].Sato S, Kitamura H, Adachi A, et al. Reduplicated basal lamina of the peritubular capillaries in renal biopsy specimens. J Submicrosc Cytol Pathol 2005;37:305–11. [PubMed] [Google Scholar]
- [17].Tan Y, Yu F, Qu Z, et al. Modified C-reactive protein might be a target autoantigen of TINU syndrome. Clin J Am Soc Nephrol 2011;6:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tan Y, Yu F, Yang H, et al. Autoantibodies against monomeric C-reactive protein in sera from patients with lupus nephritis are associated with disease activity and renal tubulointerstitial lesions. Hum Immunol 2008;69:840–4. [DOI] [PubMed] [Google Scholar]
- [19].Li QY, Li HY, Fu G, et al. Autoantibodies against C-reactive protein influence complement activation and clinical course in lupus nephritis. J Am Soc Nephrol 2017;28:3044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Espeli M, Bokers S, Giannico G, et al. Local renal autoantibody production in lupus nephritis. J Am Soc Nephrol 2011;22:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Arkatkar T, Du SW, Jacobs HM, et al. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med 2017;214:3207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chang A, Henderson SG, Brandt D, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 2011;186:1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


