Abstract
Introduction
Asthma exacerbations spike in the spring and autumn months, yet the seasonal variation of asthma symptoms and lung function is poorly studied.
Methods
Seasonal variation of lung function, rescue medication use and patient-reported symptoms was evaluated by post hoc analyses of the Phase III lebrikizumab (anti-IL-13) LAVOLTA I and II studies in 2148 subjects with uncontrolled asthma. Lung function measurements (prebronchodilator FEV1, forced vital capacity (FVC) and peak expiratory flow (PEF)), rescue medication use and Standardised Asthma Quality of Life Questionnaire (AQLQ(S)) were measured every 4 weeks over 52 weeks. By-month estimates normalised by hemispheric season were based on mixed-effect models with repeated measures (MMRM), adjusted by study stratification factors as covariates when appropriate. The dependency of clinical outcomes with seasonal variability was assessed by employing linear contrasts comparing hemisphere normalised December versus July group means from an MMRM regression and presented as the difference in means (adjusted 95% CI).
Results
FEV1, FVC and PEF, rescue medication use and AQLQ(S) progressively worsened towards winter, unlike spring and autumn surges in asthma exacerbations. The December versus July mean differences were: (1) PEF=−6.5 (–8.7 to –4.2) L/min, 2) prebronchodilator FEV1=−42 (–57 to –27) mL, (3) FVC=−41 (−59 to –23) mL and (4) AQLQ(S)=−0.15 (–0.19 to –0.1) units. Among AQLQ questions, discomfort or distress related to cough was most variable with respect to season (−0.33 (−0.42 to –0.24) units).
Discussion
Interpretation of interventional studies biased by seasonal exposures may be confounded by seasonal variability.
Trials registration numbers
NCT01867125 and NCT01868061.
Keywords: asthma, asthma epidemiology
Key messages.
Do asthma symptoms and lung function metrics vary with respect to season, similar to asthma exacerbations?
Unlike spring and autumn surges in asthma exacerbations, asthma symptoms and lung function metrics progressively worsened towards winter, though only a subset of symptoms drove the seasonal worsening of asthma symptoms.
Imbalances in symptom severity across treatment arms or bias of on-study seasonal exposure may confound the interpretation of efficacy for interventional studies.
Introduction
Epidemiological studies have shown that respiratory infections and aeroallergens precipitate seasonal increases in asthma exacerbations in the spring and autumn months. Respiratory infections and aeroallergens may trigger or amplify airway inflammation in patients with asthma, leading to increased risk of acute exacerbation events.
We have previously described the seasonal variability of asthma exacerbations and treatment effectiveness of IL-13 blockade in adults with severe, uncontrolled asthma enrolled in the LAVOLTA I and II studies.1 The LAVOLTA studies enrolled 2148 patients from 28 countries across the northern and southern hemispheres to evaluate the efficacy and safety of lebrikizumab (anti-IL-13) in adults with asthma who were uncontrolled despite inhaled corticosteroid (ICS) and a second controller therapy. The rate of severe asthma exacerbations over 52 weeks was the primary efficacy outcome. Patients were enrolled during all four seasons of the year, and more than 90% of patients completed study assessments through week 52 (LAVOLTA I, 92.2%; LAVOLTA II, 92.5%). To identify patients hypothesised to benefit most from lebrikizumab treatment, patients were classified by type 2 biomarker status (serum periostin and blood eosinophils). LAVOLTA I met its primary endpoint (rate of asthma exacerbations over 52 weeks in type 2 biomarker-high patients), but LAVOLTA II did not. In a post hoc analysis, we found that exacerbations peaked in the spring and autumn months and were at a minimum during the summer months.1 The extent to which seasonal factors influence clinical metrics of asthma symptoms such as lung function, rescue medication use and patient-reported outcomes is poorly studied. Herein, we hypothesised that lung function and symptoms may vary by season, and specifically that these measures of asthma status would be less severe in the summer months.
Methods
Patient population
We conducted post hoc analyses of LAVOLTA I and II studies (NCT01867125 and NCT01868061)2 comprising 2148 patients with severe, uncontrolled asthma that had been followed over a 52-week placebo-controlled treatment period to assess the seasonal dependence of clinical outcomes assessed every 4 weeks during the treatment period, including lung function measurements (prebronchodilator FEV1, forced vital capacity (FVC) and peak expiratory flow (PEF)), rescue medication use and Standardised Asthma Quality of Life Questionnaire (AQLQ(S)).3 Institutional Review Board or Ethics Committee approval was obtained at all participating sites and all patients provided written informed consent.
Patient and public involvement
Patients or the public were not involved in the design of the studies.
Statistical analyses
By-month estimates were based on mixed-effect models with repeated measures (MMRM), adjusted by study stratification factors as covariates as appropriate: treatment arm (placebo or lebrikizumab (anti-IL-13)), history of asthma exacerbations in the previous 12 months (0, 1 or >2), asthma controller medication at baseline (ICS dose >1000 µg of fluticasone propionate DPI or equivalent plus long-acting beta agonist (yes/no)) and geographic region (North America, Central and Eastern Europe or Rest of the World). Eleven per cent (230 of 2148) of patients were enrolled in southern hemisphere sites. To account for differences in seasons occurring in the northern and southern hemisphere, we normalised by hemispheric season, for example, 6 months was added to an observation date for patients enrolled in the southern hemisphere.
Data for each outcome were available for at least 95% of each patient’s visits over the 52 weeks; unavailable data, attributed to incomplete patient compliance, was considered Missing Completely At Random and was not imputed.
To quantify the dependency of clinical outcomes with season, linear contrasts were employed comparing hemisphere normalised December versus July group means (months were chosen empirically) from an MMRM regression and presented as the difference in means (Bonferonni adjusted 95% CI). As the treatment effect was comparable on a per-month basis, both placebo-treated and lebrikizumab-treated subjects were included in these analyses.
R software (www.r-project.org), V.3.4.3, R packages haven, lme4, lsmeans, contrast and multcomp were used for data analysis and plotting. Model diagnostics was conducted by examination of residuals versus fitted plots.
Results
We assessed the per-month adjusted mean (±SE) estimates for PEF, FEV1, FVC, rescue medication use and AQLQ(S) by treatment arm (figure 1). Group means for these outcomes were all directionally worse in winter (primarily December or January) and better in summer (June through August). The seasonal variability of these outcomes are consistent with our previous report of exacerbations, although the timing and number of peaks of these outcomes differ with exacerbations.1 Exacerbations are at a minimum in the summer and are punctuated by increase in incidence in the autumn, which is attributed to increased respiratory viral infections.1 4 5 Lung function, rescue medication use and AQLQ(S) worsening was progressive, peaking in the winter.
Figure 1.
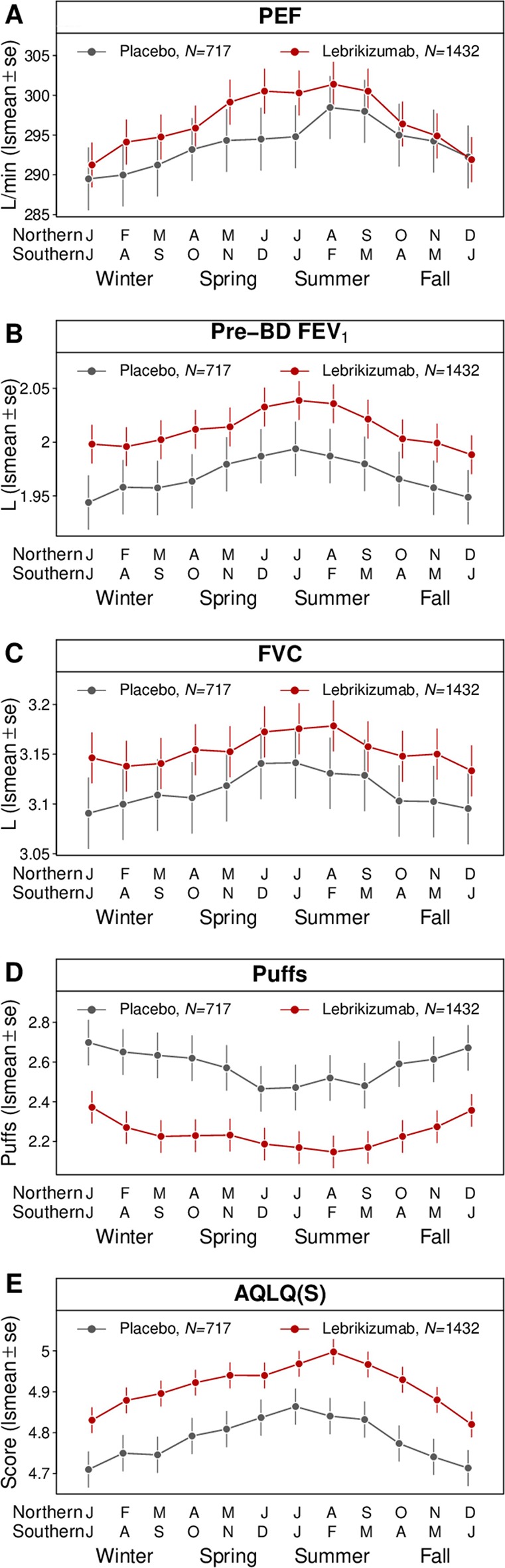
Seasonal analysis of lung function and AQLQ outcomes. Hemisphere normalised (see Methods section) per-month least-squares means (lsmeans±SE) are plotted for (A) PEF, (B) pre-BD FEV1, (C) FVC, (D) rescue medication use (puffs) and (E) AQLQ(S). AQLQ(S), Standardised Asthma Quality of Life Questionnaire Scores; BD, bronchodilator; FVC, forced vital capacity; PEF, peak expiratory flow.
While seasonal variability of exacerbations was more prominent for subjects whose blood eosinophils were greater or equal to 300/µL at baseline,1 seasonal variability of PEF, FEV1, FVC, rescue medication use and AQLQ(S) was similar regardless of patient classification by baseline blood eosinophil level (online supplementary figure 1).
bmjresp-2019-000406supp002.pdf (24KB, pdf)
The December versus July mean differences were: (1) PEF=−6.5 (−8.2 to –4.8) L/min, (2) prebronchodilator FEV1=−42 (−57 to –27) mL, (3) FVC=−41 (−59 to –23) mL, (4) rescue medication use=0.18 (0.09 to 0.28) puffs and (5) AQLQ(S)=−0.15 (−0.19 to –0.1) units (table 1). Among AQLQ questions, discomfort related to cough was most variable with respect to season (−0.33 (−0.42 to –0.24) units), whereas experiencing asthma symptoms as a result of being exposed to strong smells or perfume had the smallest seasonal effect (−0.08 (−0.16 to –0.01) units) (online supplementary table 1 and online supplementary figure 2).
Table 1.
Seasonal analysis of PEF, pre-BD FEV1, FVC, rescue medication use (puffs) and AQLQ(S)
| Difference | Lower 95% CI | Upper 95% CI | |
| PEF (L/min) | −6.5 | −8.7 | −4.2 |
| Pre-BD FEV1 (mL) | −42 | −57 | −27 |
| FVC (mL) | −41 | −59 | −23 |
| Puffs | 0.18 | 0.09 | 0.28 |
| AQLQ(S) | −0.15 | −0.19 | −0.1 |
Seasonal variability was estimated by the contrast of December and July for all subjects in the study. The differences in means with lower and upper 95% (Bonferonni adjusted) CIs are tabulated.
AQLQ(S), Standardised 32 Asthma Quality of Life Questionnaire Scores; BD, bronchodilator; FVC, forced vital capacity; PEF, peak expiratory flow.
bmjresp-2019-000406supp001.pdf (57KB, pdf)
bmjresp-2019-000406supp003.pdf (13.5KB, pdf)
Discussion
Taken together, our analyses reveal that lung function, rescue medication use and AQLQ outcomes varied with respect to season, although modestly. The strength of this study is that it was a prospective year-long global trial in a large number of patients with frequent and regular data collection. We found that all outcomes were directionally better in the summer (similar to exacerbations) and progressively worsened towards winter, in contrast to observations of spring and autumn surges in asthma exacerbations. Finally, we show variability in the seasonal dependence of individual AQLQ components, indicating that a subset of symptoms (eg, cough) drive the seasonal worsening of asthma symptoms.
In addition to the exploratory, post hoc nature of our study, there are several limitations that warrant further discussion. Minimal clinically important differences (MCID) have not been well established for PEF, rescue inhaler use or FVC. The December versus July mean difference of −42 mL for prebronchodilator FEV1 did not exceed the MCID of 230 mL described by Santanello et al.6 Likewise, the December versus July mean difference of −0.15 did not exceed the MCID of 0.5 for the AQLQ(S); however, the seasonal dependence of individual AQLQ components varied by component, which could confound analyses of the overall score. The AQLQ(S) was designed to measure the impact of asthma on quality of life in adults.3 Future studies including quantitative monitoring devices (eg, wearable cough monitors) or questionnaires tailored to measure specific patient activities are necessary to understand the contribution of seasonal variability to frequency or severity of specific patient reported outcomes. The worsening of lung function, AQLQ and rescue inhaler use in the winter suggests that respiratory infections and/or cold, dry air may trigger or amplify asthma symptoms. The lack of marked increases in the autumn and spring suggest that autumn respiratory infections and/or spring aeroallergens may not be the driving cause of lung function worsening, symptom-related quality of life deficits and rescue inhaler use. Future evaluation of asthma symptoms in the context of environmental triggers and respiratory infections are needed to provide insights into the factors that underlie these outcomes. Finally, as deterioration of lung function and worsening of asthma symptoms are integral contributors to exacerbations, it remains to be determined if the winter worsening of asthma symptoms are a cause or consequence of asthma exacerbation events.
While the observed seasonal variability for these outcomes did not exceed MCID, these data underscore the variability of asthma symptoms across season and have important implications for design of clinical studies to better evaluate efficacy for asthma symptoms; in particular, imbalances in symptom severity across treatment arms or bias of on-study seasonal exposure may confound the interpretation of efficacy.
Footnotes
Contributors: All authors participated in the design, execution, analysis and drafting of this study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: RB, XY, TS, JO, CTJH, JA and DFC are employees of Genentech, Inc and hold Roche stocks and/or options. JGM was previously an employee of Genentech at the time of this study.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available.
References
- 1.Staton TL, Arron JR, Olsson J, et al. . Seasonal variability of severe asthma exacerbations and clinical benefit from lebrikizumab. J Allergy Clin Immunol 2017;139:1682–4. 10.1016/j.jaci.2017.01.028 [DOI] [PubMed] [Google Scholar]
- 2.Hanania NA, Korenblat P, Chapman KR, et al. . Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med 2016;4:781–96. 10.1016/S2213-2600(16)30265-X [DOI] [PubMed] [Google Scholar]
- 3.Juniper EF, Buist AS, Cox FM, et al. . Validation of a standardized version of the asthma quality of life questionnaire. Chest 1999;115:1265–70. 10.1378/chest.115.5.1265 [DOI] [PubMed] [Google Scholar]
- 4.Johnston NW, Johnston SL, Norman GR, et al. . The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol 2006;117:557–62. 10.1016/j.jaci.2005.11.034 [DOI] [PubMed] [Google Scholar]
- 5.Busse WW, Morgan WJ, Gergen PJ, et al. . Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011;364:1005–15. 10.1056/NEJMoa1009705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santanello NC, Zhang J, Seidenberg B, et al. . What are minimal important changes for asthma measures in a clinical trial? Eur Respir J 1999;14:23–7. 10.1034/j.1399-3003.1999.14a06.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2019-000406supp002.pdf (24KB, pdf)
bmjresp-2019-000406supp001.pdf (57KB, pdf)
bmjresp-2019-000406supp003.pdf (13.5KB, pdf)


