Figure 5.
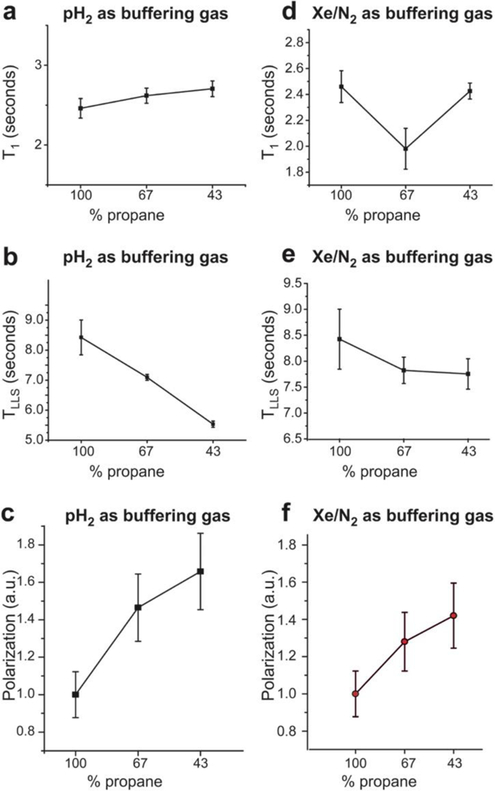
Effects of using different buffering gases on the hyperpolarization decay constants and polarization levels of HP propane determined using the 0.0475 T magnetic field NMR spectrometer setup (Scheme 1). T1 (a) and TLLS (b) dependence of HP propane on the propane fraction in the resultant gas mixtures with the use of pH2 buffering gas. c) Dependence of propane 1H polarization (arbitrary units, a.u.) on the propane fraction in the resultant gas mixtures with the use of H2 buffering gas. T1 (d) and TLLS (e) dependence of HP propane on the propane fraction in the resultant gas mixtures with the use of Xe/N2 (3:1) buffering gas mixture. f) Dependence of propane 1H polarization (arbitrary units, a.u.) on the propane fraction in the resultant gas mixtures with the use of Xe/N2 (3:1) buffering gas mixture. All data were obtained at 38 psi backpressure (~3.6 atm total pressure), and the pH2 to propylene ratio was 1:1 for the experiments shown in displays d, e, and f. Connecting lines are meant only to guide the eye.
